Segetalin ACAS# 161875-97-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 161875-97-4 | SDF | Download SDF |
| PubChem ID | 10483858 | Appearance | Powder |
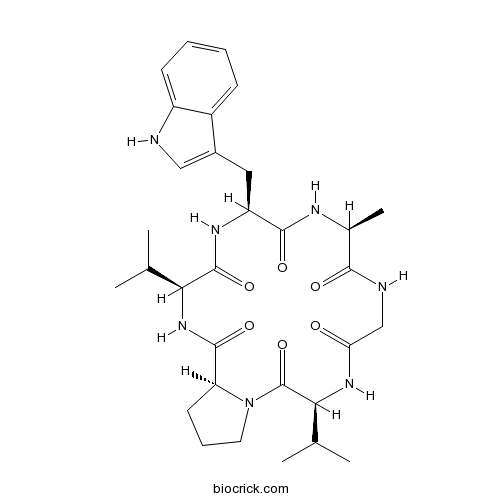
| Formula | C31H43N7O6 | M.Wt | 609.7 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,9S,12S,15S,18S)-12-(1H-indol-3-ylmethyl)-9-methyl-3,15-di(propan-2-yl)-1,4,7,10,13,16-hexazabicyclo[16.3.0]henicosane-2,5,8,11,14,17-hexone | ||
| SMILES | CC1C(=O)NCC(=O)NC(C(=O)N2CCCC2C(=O)NC(C(=O)NC(C(=O)N1)CC3=CNC4=CC=CC=C43)C(C)C)C(C)C | ||
| Standard InChIKey | YVUZOKYOUUCVBV-WJTDBPPMSA-N | ||
| Standard InChI | InChI=1S/C31H43N7O6/c1-16(2)25-30(43)35-22(13-19-14-32-21-10-7-6-9-20(19)21)28(41)34-18(5)27(40)33-15-24(39)36-26(17(3)4)31(44)38-12-8-11-23(38)29(42)37-25/h6-7,9-10,14,16-18,22-23,25-26,32H,8,11-13,15H2,1-5H3,(H,33,40)(H,34,41)(H,35,43)(H,36,39)(H,37,42)/t18-,22-,23-,25-,26-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Segetalin A has estrogen-like activity. |
| Targets | Estrogen receptor | Progestogen receptor |

Segetalin A Dilution Calculator

Segetalin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6402 mL | 8.2008 mL | 16.4015 mL | 32.803 mL | 41.0038 mL |
| 5 mM | 0.328 mL | 1.6402 mL | 3.2803 mL | 6.5606 mL | 8.2008 mL |
| 10 mM | 0.164 mL | 0.8201 mL | 1.6402 mL | 3.2803 mL | 4.1004 mL |
| 50 mM | 0.0328 mL | 0.164 mL | 0.328 mL | 0.6561 mL | 0.8201 mL |
| 100 mM | 0.0164 mL | 0.082 mL | 0.164 mL | 0.328 mL | 0.41 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Talampanel(LY300164)
Catalog No.:BCC6378
CAS No.:161832-65-1
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Benzamil
Catalog No.:BCC7674
CAS No.:161804-20-2
- Ethyl 2-(3-cyano-4-hydroxyphenyl)-4-methyl-1,3-thiazole-5-carboxylate
Catalog No.:BCC8966
CAS No.:161798-02-3
- Ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
Catalog No.:BCC8968
CAS No.:161798-01-2
- Ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
Catalog No.:BCC8969
CAS No.:161797-99-5
- Esomeprazole Sodium
Catalog No.:BCC4376
CAS No.:161796-78-7
- Palmatrubine
Catalog No.:BCN2647
CAS No.:16176-68-4
- 12-Oxocalanolide A
Catalog No.:BCN4699
CAS No.:161753-49-7
- GLP-1 (9-36) amide
Catalog No.:BCC6001
CAS No.:161748-29-4
- Rasagiline mesylate
Catalog No.:BCN2166
CAS No.:161735-79-1
- Tebipenempivoxil
Catalog No.:BCC3861
CAS No.:161715-24-8
- Prehelminthosporol
Catalog No.:BCN7447
CAS No.:1619-13-2
- Esomeprazole Magnesium
Catalog No.:BCC5007
CAS No.:161973-10-0
- Catechin pentaacetate
Catalog No.:BCN1718
CAS No.:16198-01-9
- LY2857785
Catalog No.:BCC8050
CAS No.:1619903-54-6
- GSK2801
Catalog No.:BCC6498
CAS No.:1619994-68-1
- Bromosporine
Catalog No.:BCC2226
CAS No.:1619994-69-2
- Rofecoxib
Catalog No.:BCC4437
CAS No.:162011-90-7
- UNC0379
Catalog No.:BCC8055
CAS No.:1620401-82-2
- L-371,257
Catalog No.:BCC7353
CAS No.:162042-44-6
- SC 58125
Catalog No.:BCC5948
CAS No.:162054-19-5
- Myriceric acid C
Catalog No.:BCN1719
CAS No.:162059-94-1
- Dimesna
Catalog No.:BCC1095
CAS No.:16208-51-8
A sensitive and selective LC-MS/MS method for the quantitative determination of segetalin A from the plasma of rats.[Pubmed:26295277]
Biomed Chromatogr. 2016 Apr;30(4):606-11.
A sensitive and selective LC-MS/MS method was developed and validated for the determination and pharmacokinetic investigation of Segetalin A in rat plasma. Sample preparation was accomplished through a simple SPE procedure for the removal and preconcentration of the analyte and IS. Plasma samples were separated by HPLC on a Symmetry C18 column using a mobile phase consisting of methanol and 0.1% formic acid in water (70:30, v/v) with isocratic elution. The quantification was performed using multiple reaction monitoring with the transitions m/z 610.3 --> 511.2 for Segetalin A and m/z 779.4 --> 751.4 for IS, respectively. The calibration curve was linear over the range of 8.0-4000 ng/mL with a limit of quantitation (LOQ) of 8.0 ng/mL. This method was applied in a pharmacokinetic study of Segetalin A in rats. For intravenous (i.v.) administration, the plasma concentrations of Segetalin A decreased quickly (t1/2z, 1.31 +/- 0.341 h). For oral administration, the plasma concentrations of Segetalin A increased to a peak value at 1.50 +/- 0.577 h, followed by a gradual decrease to the LOQ in 12 h. The mean AUC values after i.v. and oral administration were 553 +/- 105 and 1482 +/- 110 ng h/mL, respectively.
Antiproliferative activity of Saponaria vaccaria constituents and related compounds.[Pubmed:22056663]
Fitoterapia. 2012 Jan;83(1):170-81.
Total methanolic extracts of Saponaria vaccaria seed derived from several varieties, as well as various purified components obtained through successive chromatographic separations of total extracts were evaluated for their growth inhibitory activity in WiDr (colon), MDA-MB-231 (breast), NCI-417 (lung) and PC-3 (prostate) human cancer cells as well as the non-tumorigenic fibroblast BJ (CRL-2522) cell line using MTT colorimetric assay. Purified bisdesmosidic saponins segetoside H and I were further examined using microscopy and apoptosis assays. Bisdesmosidic saponins exhibited dose-dependent growth inhibitory and selective apoptosis-inducing activity. Growth inhibitory effects were particularly strong in a breast (MDA-MB-231) and a prostate (PC-3) cancer cell line. Total extracts exhibited a different preference being most active against a colon cancer cell line (WiDr). In a comparison of varieties, all of the total seed extracts exhibited similar dose-dependent activities, but with some variation in potency. Monodesmosidic saponins vaccarosides A and B, phenolic vaccarin, and cyclopeptide Segetalin A, co-occurring seed substituents, did not exhibit activity. The non-tumorigenic fibroblast cell line BJ (CRL 2522) was growth inhibited but did not undergo apoptosis when treated with bisdesmosidic saponins at low micromolar concentrations. Saponin-rich extracts from Kochia scoparia seed and Chenopodium quinoa were also evaluated alongside Saponaria saponins but did not exhibit activity. Closely related Quillaja saponins exhibited activity but were less potent.
The biosynthesis of Caryophyllaceae-like cyclic peptides in Saponaria vaccaria L. from DNA-encoded precursors.[Pubmed:21554452]
Plant J. 2011 Aug;67(4):682-90.
Cyclic peptides (CPs) are produced in a very wide range of taxa. Their biosynthesis generally involves either non-ribosomal peptide synthases or ribosome-dependent production of precursor peptides. Plants within the Caryophyllaceae and certain other families produce CPs which generally consist of 5-9 proteinogenic amino acids. The biological roles for these CPs in the plant are not very clear, but many of them have activity in mammalian systems. There is currently very little known about the biosynthesis of CPs in the Caryophyllaceae. A collection of expressed sequence tags from developing seeds of Saponaria vaccaria was investigated for information about CP biosynthesis. This revealed genes that appeared to encode CP precursors which are subsequently cyclized to mature CPs. This was tested and confirmed by the expression of a cDNA encoding a putative precursor of the CP Segetalin A in transformed S. vaccaria roots. Similarly, extracts of developing S. vaccaria seeds were shown to catalyze the production of Segetalin A from the same putative (synthetic) precursor. Moreover, the presence in S. vaccaria seeds of two segetalins, J [cyclo(FGTHGLPAP)] and K [cyclo(GRVKA)], which was predicted by sequence analysis, was confirmed by liquid chromatography/mass spectrometry. Sequence analysis also predicts the presence of similar CP precursor genes in Dianthus caryophyllus and Citrus spp. The data support the ribosome-dependent biosynthesis of Caryophyllaceae-like CPs in the Caryophyllaceae and Rutaceae.
Application of high-speed counter-current chromatography for preparative separation of cyclic peptides from Vaccaria segetalis.[Pubmed:21396894]
J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Apr 1;879(11-12):811-4.
Following an initial clean-up step on silica gel, high-speed counter-current chromatography (HSCCC) was used to separate cyclic peptides from an extract of the seeds of Vaccaria segetalis. The two-phase solvent system used for HSCCC separation was composed of petroleum ether-ethyl acetate-methanol-water at an optimized volume ratio of 0.5:3.5:1:5. From 190 mg of crude extract, 38.0 mg of segetalin B and 28.5 mg of Segetalin A were obtained with purities of 98.1% and 95.6% as determined by HPLC, respectively. The chemical structures of the target compounds were confirmed by high resolution electrospray ionization time of flight MS (HRESI-TOF-MS) and (1)H NMR analyses.
Thionation of segetalins A and B, cyclic peptides with estrogen-like activity from seeds of Vaccaria segetalis.[Pubmed:9113340]
Bioorg Med Chem. 1997 Mar;5(3):631-6.
Thionation of estrogen-like active cyclic peptides, segetalins A (1) and B (2), with Lawesson's reagent provided each two thiosegetalins; thioSegetalin A1 [Gly-1-psi(CS-NH)-Val-2; Trp-5-psi (CS-NH)-Ala-6]Segetalin A, thioSegetalin A2 [Gly-1-psi(CS-NH)-Val-2; Ala-6-psi(CS-NH)-Gly-1]Segetalin A, thiosegetalin B1 [Gly-1-psi (CS-NH)-Val-2; Ala-3-psi(CS-NH)-Trp-4]segetalin B, and thiosegetalin B2 [Gly-1-psi(CS-NH)-Val-2; Trp-4-psi(CS-NH)-Ala-1]segetalin B. ThioSegetalin A2 only showed estrogen-like activity against ovariectomized rats. On the basis of their conformations analysed by NMR experiments, the backbone conformation was considered to play an important role in estrogen-like activity for segetalins.


