SC 58125Selective cyclooxygenase-2 (COX-2) inhibitor CAS# 162054-19-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 162054-19-5 | SDF | Download SDF |
| PubChem ID | 115239 | Appearance | Powder |
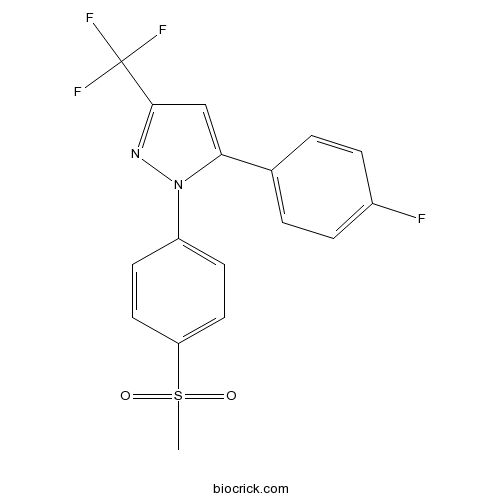
| Formula | C17H12F4N2O2S | M.Wt | 384.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
| Chemical Name | 5-(4-fluorophenyl)-1-(4-methylsulfonylphenyl)-3-(trifluoromethyl)pyrazole | ||
| SMILES | CS(=O)(=O)C1=CC=C(C=C1)N2C(=CC(=N2)C(F)(F)F)C3=CC=C(C=C3)F | ||
| Standard InChIKey | JHBIMJKLBUMNAU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H12F4N2O2S/c1-26(24,25)14-8-6-13(7-9-14)23-15(10-16(22-23)17(19,20)21)11-2-4-12(18)5-3-11/h2-10H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective cyclooxygenase 2 (COX-2) inhibitor (IC50 values are 0.04 and >100 μM for COX-2 and COX-1 respectively). Anti-inflammatory; blocks edema and hyperalgesia in vivo following an inflammatory insult, without causing gastric mucosal damage. Also displays antitumor activity. |

SC 58125 Dilution Calculator

SC 58125 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6018 mL | 13.009 mL | 26.018 mL | 52.0359 mL | 65.0449 mL |
| 5 mM | 0.5204 mL | 2.6018 mL | 5.2036 mL | 10.4072 mL | 13.009 mL |
| 10 mM | 0.2602 mL | 1.3009 mL | 2.6018 mL | 5.2036 mL | 6.5045 mL |
| 50 mM | 0.052 mL | 0.2602 mL | 0.5204 mL | 1.0407 mL | 1.3009 mL |
| 100 mM | 0.026 mL | 0.1301 mL | 0.2602 mL | 0.5204 mL | 0.6504 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- L-371,257
Catalog No.:BCC7353
CAS No.:162042-44-6
- UNC0379
Catalog No.:BCC8055
CAS No.:1620401-82-2
- Rofecoxib
Catalog No.:BCC4437
CAS No.:162011-90-7
- Bromosporine
Catalog No.:BCC2226
CAS No.:1619994-69-2
- GSK2801
Catalog No.:BCC6498
CAS No.:1619994-68-1
- LY2857785
Catalog No.:BCC8050
CAS No.:1619903-54-6
- Catechin pentaacetate
Catalog No.:BCN1718
CAS No.:16198-01-9
- Esomeprazole Magnesium
Catalog No.:BCC5007
CAS No.:161973-10-0
- Prehelminthosporol
Catalog No.:BCN7447
CAS No.:1619-13-2
- Segetalin A
Catalog No.:BCC9246
CAS No.:161875-97-4
- Talampanel(LY300164)
Catalog No.:BCC6378
CAS No.:161832-65-1
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Myriceric acid C
Catalog No.:BCN1719
CAS No.:162059-94-1
- Dimesna
Catalog No.:BCC1095
CAS No.:16208-51-8
- 4',4'''-Di-O-methylisochamaejasmin
Catalog No.:BCN6849
CAS No.:1620921-68-7
- SynaptoRedTM C2
Catalog No.:BCC8012
CAS No.:162112-35-8
- RWJ 50271
Catalog No.:BCC7894
CAS No.:162112-37-0
- Flunitrazepam
Catalog No.:BCC6107
CAS No.:1622-62-4
- Melperone hydrochloride
Catalog No.:BCC7385
CAS No.:1622-79-3
- 5'-Deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine 2',3'-diacetate
Catalog No.:BCN1544
CAS No.:162204-20-8
- Dorsmanin A
Catalog No.:BCN4088
CAS No.:162229-27-8
- CI 1020
Catalog No.:BCC7523
CAS No.:162256-50-0
- 7,3'-Dihydroxy-4'-methoxyflavan
Catalog No.:BCN4698
CAS No.:162290-05-3
- Remodelin
Catalog No.:BCC5571
CAS No.:1622921-15-6
Inhibitory effects of Celecoxib and Sc-58125 on proliferation of human carcinoma of larynx Hep-2 in vitro.[Pubmed:16116973]
J Huazhong Univ Sci Technolog Med Sci. 2005;25(2):202-5.
The inhibitory effects of two kinds of selective cyclooxygenase-2 inhibitors on the proliferation of human carcinoma of larynx Hep-2 in vitro and their corresponding mechanisms were investigated. Hep-2 cells were cultured with two kinds of selective cyclooxygenase-2 inhibitors (Sc-58125 and Celecoxib) at various concentrations for 24 h. Morphological changes were observed under the phase microscopy and the growth suppression was detected by using MTT colorimetric assay. Apoptotic DNA fragments were observed by agarose gel electrophoresis, and the cell cycle and apoptotic rate were detected by flow cytometry (FCM) respectively. Hep-2 cells became rounded and detached from the culture dish after being treated with Celecoxib for 24 h, however, they remained morphologically unchanged with Sc-58125. Sc-58125 could increase G2 phase cells, whereas, Celecoxib rose G1 phase cells. Both of the two effects were dose-dependent. Moreover, the Hep-2 cells cultured with 50 micromol/L and 100 micromol/L Celecoxib showed obvious apoptosis, with the nuclear DNA of cells exhibiting characteristic DNA ladder. So Sc-58125 could inhibit the proliferation of Hep-2 cells by altering the G2 phase cells. However, Celecoxib had the same effect by changing the G1 phase cells and inducing apoptosis at higher concentration.
Aspirin, but not the more selective cyclooxygenase (COX)-2 inhibitors meloxicam and SC 58125, aggravates postischaemic cardiac dysfunction, independent of COX function.[Pubmed:11218076]
Naunyn Schmiedebergs Arch Pharmacol. 2001 Feb;363(2):233-40.
Inhibition of cyclooxygenase (COX) might favour non-enzymatic formation of cardiodepressive isoprostanes from arachidonic acid by radicals generated during reperfusion. This could explain deleterious effects of acetylsalicylic acid (ASA) on cardiac function. We examined the influence of COX inhibition on myocardial function after low-flow ischaemia and reperfusion, employing either ASA (100 micromol/l), the partially selective COX-2 inhibitor meloxicam (0.3 micromol/l and 3.0 micromol/l), or the highly selective COX-2 inhibitor SC 58125 (1.0 micromol/l and 3.0 microgmol/l). Isolated, buffer-perfused guinea pig hearts, performing pressure-volume work before and after consecutive low-flow ischaemia and reperfusion, were used for the study. Measurement of coronary and aortic flow, ejection time and heart rate served to calculate external heart work (EHW), before and after ischaemia. Additionally, release of prostacyclin and thromboxane A2, production of lactate, consumption of pyruvate and tissue concentration of the isoprostane 8-iso-PGF2alpha were measured. ASA significantly reduced recovery of EHW (46+/-18% vs. 82+/-15% for controls), whereas meloxicam and SC 58125 did not (64+/-15% and 74+/-13% recovery, respectively). Paradoxically, ASA increased reactive hyperaemia and consumption of pyruvate in the early reperfusion phase in comparison to all other groups, while lactate production did not differ. Prostacyclin production did not increase during reperfusion and was not significantly different between groups at any time point. In contrast, thromboxane A2 release increased about fivefold in the 2nd min of reperfusion under control conditions and in the presence of SC 58125, but was inhibited by ASA and by meloxicam in both concentrations. Isoprostane content of heart tissue was not detectably influenced under the mild reperfusion conditions used here. We conclude that ASA can aggravate postischaemic cardiac dysfunction, independent of COX inhibition. The deleterious effect in the present model might be due to uncoupling of mitochondrial oxidative phosphorylation rather than to direct effects of reduced eicosanoid release or radical induced formation of isoprostanes.
A cyclooxygenase-2 inhibitor (SC-58125) blocks growth of established human colon cancer xenografts.[Pubmed:11687954]
Neoplasia. 2001 Sep-Oct;3(5):428-36.
Selective COX-2 inhibitors reduce adenoma formation and cancer progression in rodent models of colorectal cancer. To assess the therapeutic activity of selective COX-2 inhibitors, we tested the effect of SC-58125 treatment on the growth of human colon carcinoma cells in nude mice. Delaying treatment by 2, 4, or 7 weeks following implantation of the carcinoma cells resulted in a significant inhibition of tumor growth. Furthermore, short-term (48 hours) treatment with SC-58125 was sufficient to attenuate tumor growth for up to 15 days. SC-58125 treatment did not alter the rate at which cells underwent apoptosis, but did result in a delayed progression through the cell cycle at the G(2)/M transition. Accordingly, p34(cdc2) protein levels and activity were decreased following SC-58125 treatment. We conclude that SC-58125 primarily exerts a cytostatic effect in vivo, which is likely to be mediated through inhibition of progression through the G(2)/M phase of the cell cycle.
A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors.[Pubmed:8663121]
J Biol Chem. 1996 Jun 28;271(26):15810-4.
Nonsteroidal anti-inflammatory drugs (NSAIDs) currently available for clinical use inhibit both COX-1 and COX-2. This suggests that clinically useful NSAIDs inhibit pro-inflammatory prostaglandins (PGs) derived from the activity of COX-2, as well as PGs in tissues like the stomach and kidney (via COX-1). A new class of compounds has recently been developed (SC-58125) that have a high degree of selectivity for the inducible form of cyxlooxygenase (COX-2) over the constitutive form (COX-1). This unique class of compounds exhibit a time-dependent irreversible inhibition of COX-2, while reversibly inhibiting COX-1. The molecular basis of this selectivity was probed by site-directed mutagenesis of the active site of COX-2. The sequence differences in the active site were determined by amino acid replacement of the COX-2 sequences based on the known crystal structure of COX-1, which revealed a single amino acid difference in the active site (valine 509 to isoleucine) and a series of differences at the mouth of the active site. Mutants with the single amino acid substitution in the active site and a combination of three changes in the mouth of the active site were made in human COX-2, expressed in insect cells and purified. The single amino acid change of valine 509 to isoleucine confers selectivity of COX-2 inhibitors in the class of SC-58125 and others of the same class (SC-236, NS-398), while commonly used NSAIDs such as indomethacin showed no change in selectivity. Substitutions of COX-1 sequences in COX-2 at the mouth of the active site of COX-2 did not change the selectivity of SC-58125. This indicates that the single amino acid substitution of isoleucine at position 509 for a valine is sufficient to confer COX-2 selectivity in this example of a diaryl-heterocycle COX inhibitor.


