MyrislignanCAS# 171485-39-5 |
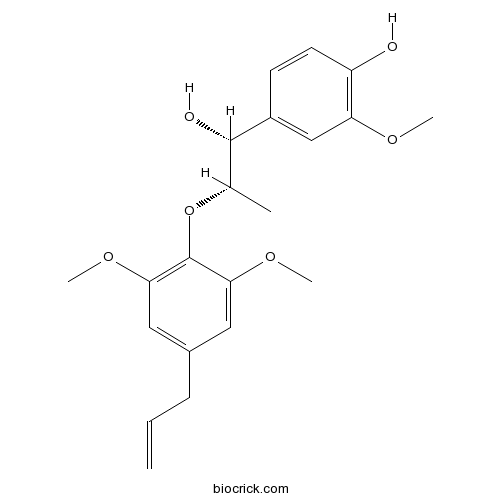
- 1-(3,4-dimethoxyphenyl)-2-(4-allly-2,6-dimethoxyphenoxy)propan-1-ol
Catalog No.:BCN1445
CAS No.:41535-95-9
- cis-Myrislignan
Catalog No.:BCX1556
CAS No.:52190-21-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 171485-39-5 | SDF | Download SDF |
| PubChem ID | 21636106 | Appearance | White powder |
| Formula | C21H26O6 | M.Wt | 374.43 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(1R,2S)-2-(2,6-dimethoxy-4-prop-2-enylphenoxy)-1-hydroxypropyl]-2-methoxyphenol | ||
| SMILES | CC(C(C1=CC(=C(C=C1)O)OC)O)OC2=C(C=C(C=C2OC)CC=C)OC | ||
| Standard InChIKey | ULZFTGWWPHYLGI-RBZFPXEDSA-N | ||
| Standard InChI | InChI=1S/C21H26O6/c1-6-7-14-10-18(25-4)21(19(11-14)26-5)27-13(2)20(23)15-8-9-16(22)17(12-15)24-3/h6,8-13,20,22-23H,1,7H2,2-5H3/t13-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Myrislignan has anti-inflammatory, and anti-cancer activities, it inhibited NF-κB signalling pathway activation, inhibited the proliferation of A549 cells in a dose- and time-dependent manner, it also significantly induced apoptosis and cell cycle arrest in A549 cells. |
| Targets | NO | TNF-α | NOS | COX | NF-kB | IkB | Bcl-2/Bax | c-Myc | IKK |
| In vitro | The action and mechanism of myrislignan on A549 cells in vitro and in vivo.[Pubmed: 27491743 ]J Nat Med. 2017 Jan;71(1):76-85.Myrislignan is a natural compound with little pharmacological study. In our investigation, we investigated the effect of Myrislignan in the induction of apoptosis in A549 cells in vitro and in vivo.
|
| Kinase Assay | New neolignans from the seeds of Myristica fragrans that inhibit nitric oxide production.[Pubmed: 25466017]Food Chem. 2015 Apr 15;173:231-7.Five new 8-O-4' type neolignans, named myrifralignan A-E (1-5), together with five known analogues (6-10), were isolated from the seeds of Myristica fragrans Houtt.
|
| Cell Research | Myrislignan attenuates lipopolysaccharide-induced inflammation reaction in murine macrophage cells through inhibition of NF-κB signalling pathway activation.[Pubmed: 22294521]Phytother Res. 2012 Sep;26(9):1320-6.Myrislignan is a new kind of lignan isolated from Myristica fragrans Houtt. Its antiinflammatory effects have not yet been reported.
|
| Structure Identification | Biomed Chromatogr. 2008 Jun;22(6):601-5.Quantification of myrislignan in rat plasma by solid-phase extraction and reversed-phase high-performance liquid chromatography.[Pubmed: 18254153]
|

Myrislignan Dilution Calculator

Myrislignan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6707 mL | 13.3536 mL | 26.7073 mL | 53.4145 mL | 66.7682 mL |
| 5 mM | 0.5341 mL | 2.6707 mL | 5.3415 mL | 10.6829 mL | 13.3536 mL |
| 10 mM | 0.2671 mL | 1.3354 mL | 2.6707 mL | 5.3415 mL | 6.6768 mL |
| 50 mM | 0.0534 mL | 0.2671 mL | 0.5341 mL | 1.0683 mL | 1.3354 mL |
| 100 mM | 0.0267 mL | 0.1335 mL | 0.2671 mL | 0.5341 mL | 0.6677 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Salprionin
Catalog No.:BCN3162
CAS No.:171439-43-3
- Dammarenediol II 3-O-caffeate
Catalog No.:BCN6519
CAS No.:171438-55-4
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- Otophylloside B 4'''-O-beta-D-cymaropyranoside
Catalog No.:BCN7522
CAS No.:171422-85-8
- Otophylloside B 4'''-O-alpha-L-cymaropyranoside
Catalog No.:BCN7511
CAS No.:171422-82-5
- Calcium Gluceptate
Catalog No.:BCC3743
CAS No.:17140-60-2
- Ethyl 4-hydroxyphenylacetate
Catalog No.:BCN3792
CAS No.:17138-28-2
- Delphinidin-3-O-arabinoside chloride
Catalog No.:BCN3021
CAS No.:171370-55-1
- (RS)-AMPA hydrobromide
Catalog No.:BCC6926
CAS No.:171259-81-7
- TAPI-1
Catalog No.:BCC5400
CAS No.:171235-71-5
- Posaconazole
Catalog No.:BCC1103
CAS No.:171228-49-2
- N-Methylquipazine dimaleate
Catalog No.:BCC6697
CAS No.:171205-17-7
- Alexidine dihydrochloride
Catalog No.:BCC2466
CAS No.:1715-30-6
- Urocortin (rat)
Catalog No.:BCC5789
CAS No.:171543-83-2
- 10,11-Dihydro-24-hydroxyaflavinine
Catalog No.:BCN7440
CAS No.:171569-81-6
- Doederleinic Acid
Catalog No.:BCC8319
CAS No.:171596-14-8
- Tadalafil
Catalog No.:BCC2281
CAS No.:171596-29-5
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- NBI-98854
Catalog No.:BCC4278
CAS No.:171598-74-6
- Sildenafil Citrate
Catalog No.:BCC2276
CAS No.:171599-83-0
- Sodium Phenylbutyrate
Catalog No.:BCC2164
CAS No.:1716-12-7
- Nitidanin
Catalog No.:BCN1107
CAS No.:171674-89-8
- Compound 56
Catalog No.:BCC3615
CAS No.:171745-13-4
- S-Adenosyl-L-Methtonine
Catalog No.:BCN2231
CAS No.:17176-17-9
New neolignans from the seeds of Myristica fragrans that inhibit nitric oxide production.[Pubmed:25466017]
Food Chem. 2015 Apr 15;173:231-7.
Five new 8-O-4' type neolignans, named myrifralignan A-E (1-5), together with five known analogues (6-10), were isolated from the seeds of Myristica fragrans Houtt. Their chemical structures were determined using several spectroscopic methods. Compounds 3-10 exhibited potent inhibitory activity against the production of nitric oxide (NO) in the RAW264.7 cell line stimulated by lipopolysaccaride. Myrislignan (7) and machilin D (10) were the most potent inhibitors of NO production amongst these compounds. The IC50 values of Myrislignan and machilin D were 21.2 and 18.5 muM. And, their inhibitory activity was more than L-N(6)-(1-iminoethyl)-lysine, a selective inhibitor of inducible nitric oxide synthase (IC50=27.1 muM). Furthermore, real-time PCR analysis revealed that these neolignans could significantly suppress the expression of inducible nitric oxide synthase mRNA. These results demonstrated that the 8-O-4' type neolignans are promising candidates as anti-inflammatory agents.
Myrislignan attenuates lipopolysaccharide-induced inflammation reaction in murine macrophage cells through inhibition of NF-kappaB signalling pathway activation.[Pubmed:22294521]
Phytother Res. 2012 Sep;26(9):1320-6.
Myrislignan is a new kind of lignan isolated from Myristica fragrans Houtt. Its antiinflammatory effects have not yet been reported. In the present study, the antiinflammatory effects and the underlying mechanisms of Myrislignan in lipopolysaccharide (LPS)-induced inflammation in murine RAW 264.7 macrophage cells were investigated. Myrislignan significantly inhibited LPS-induced production of nitric oxide (NO) in a dose-dependent manner. It inhibited mRNA expression and release of interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-alpha). This compound significantly inhibited mRNA and protein expressions of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) dose-dependently in LPS-stimulated macrophage cells. Further study showed that Myrislignan decreased the cytoplasmic loss of inhibitor kappaB-alpha (IkappaB-alpha) protein and the translocation of NF-kappaB from cytoplasm to the nucleus. Our results suggest that Myrislignan may exert its antiinflammatory effects in LPS-stimulated macrophages cells by inhibiting the NF-kappaB signalling pathway activation.
The action and mechanism of myrislignan on A549 cells in vitro and in vivo.[Pubmed:27491743]
J Nat Med. 2017 Jan;71(1):76-85.
Myrislignan is a natural compound with little pharmacological study. In our investigation, we investigated the effect of Myrislignan in the induction of apoptosis in A549 cells in vitro and in vivo. Myrislignan inhibited the proliferation of A549 cells in a dose- and time-dependent manner assayed by MTT. In addition, Hoechst flow cytometry showed that Myrislignan significantly induced apoptosis and cell cycle arrest in A549 cells. The apoptosis and anti-cell proliferation was mediated by the activation of mitogen-activated protein kinase and the inhibition of epidermal growth factor receptor signal pathway, change of mitochondrial membrane potential, the releasing of c-Myc, the downregulation of the level of the anti-apoptotic protein Bcl-2, and the upregulation of the level of the pro-apoptotic protein Bax. In conclusion, those results reveal a potential mechanism for the anti-cancer effect of Myrislignan on human lung cancer, while suggesting that Myrislignan may be a promising compound for the treatment of lung cancer.
Quantification of myrislignan in rat plasma by solid-phase extraction and reversed-phase high-performance liquid chromatography.[Pubmed:18254153]
Biomed Chromatogr. 2008 Jun;22(6):601-5.
A rapid and simple reversed-phase high-performance liquid chromatographic (RP-HPLC) method has been developed for determination of Myrislignan in rat plasma after intravenous administration. The analytes extracted from plasma samples by solid-phase extraction were successfully carried out on a Diamonsiltrade mark ODS C(18) column (250 x 4.6 mm i.d., 5 microm) with an RP(18) guard column (8 x 4.6 mm i.d., 5 microm) and a mobile phase of MeOH-H(2)O (4:1, v/v). The UV detector was set at a single wavelength of 270 nm. The linear ranges of the standard curves were 0.5-30.0 microg/mL with the correlation coefficients greater than 0.9992. The lower limits of detection and quantification were 0.1 and 0.3 microg/mL for Myrislignan. Intra- and inter-day precisions were 2.4-7.5 and 1.3-5.7%, respectively. The extraction recovery from plasma was more than 90%. This assay method has been successfully used to study the pharmacokinetics of Myrislignan in rats.


