TAPI-1TACE/ADAM17 inhibitor CAS# 171235-71-5 |
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Azaphen dihydrochloride monohydrate
Catalog No.:BCC1391
CAS No.:63302-99-8
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 171235-71-5 | SDF | Download SDF |
| PubChem ID | 9827273 | Appearance | Powder |
| Formula | C26H37N5O5 | M.Wt | 499.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
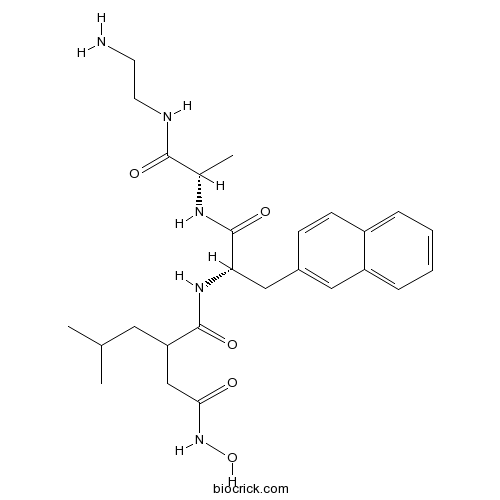
| Chemical Name | N-[(2S)-1-[[(2S)-1-(2-aminoethylamino)-1-oxopropan-2-yl]amino]-3-naphthalen-2-yl-1-oxopropan-2-yl]-N'-hydroxy-2-(2-methylpropyl)butanediamide | ||
| SMILES | CC(C)CC(CC(=O)NO)C(=O)NC(CC1=CC2=CC=CC=C2C=C1)C(=O)NC(C)C(=O)NCCN | ||
| Standard InChIKey | AWNBSWDIOCXWJW-OWHMDLSXSA-N | ||
| Standard InChI | InChI=1S/C26H37N5O5/c1-16(2)12-21(15-23(32)31-36)25(34)30-22(26(35)29-17(3)24(33)28-11-10-27)14-18-8-9-19-6-4-5-7-20(19)13-18/h4-9,13,16-17,21-22,36H,10-12,14-15,27H2,1-3H3,(H,28,33)(H,29,35)(H,30,34)(H,31,32)/t17-,21?,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TAPI-1 is an inhibitor of ADAM17/TACE. | |||||
| Targets | ADAM17/TACE | |||||

TAPI-1 Dilution Calculator

TAPI-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0016 mL | 10.008 mL | 20.016 mL | 40.032 mL | 50.04 mL |
| 5 mM | 0.4003 mL | 2.0016 mL | 4.0032 mL | 8.0064 mL | 10.008 mL |
| 10 mM | 0.2002 mL | 1.0008 mL | 2.0016 mL | 4.0032 mL | 5.004 mL |
| 50 mM | 0.04 mL | 0.2002 mL | 0.4003 mL | 0.8006 mL | 1.0008 mL |
| 100 mM | 0.02 mL | 0.1001 mL | 0.2002 mL | 0.4003 mL | 0.5004 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TAPI-1 is an inhibitor of tumour necrosis factor with IC50 value of 8.09 μM [1].
TAPI-1 is an inhibitor of TACE/ADAM17 which catalyzes the cleavage of full-length APP to the soluble N-terminal fragment (sAPPα). The release of sAPPα has been reported to be a receptor-coupled process increased by the stimulation of muscarinic receptors. In HEK293 cells, treatment of TAPI-1 resulted in an inhibition of increased sAPPα which was induced by the expressing of M3 subtype. The IC50 values of TAPI-1 in the inhibition of M3-increased sAPPα and constitutive release of sAPPα were 3.61 μM and 8.09 μM, respectively. Besides APP, TAPI-1 was also found to have inhibitory effects on the release of TNF-α, IL6R, TNFRI and TNFRII with IC50 values of 50-100, 5-10, 5-10 and 25-50 mM, respectively [1, 2].
References:
[1] Slack B, Ma L, Seah C. Constitutive shedding of the amyloid precursor protein ectodomain is up-regulated by tumour necrosis factor-α converting enzyme. Biochem. J, 2001, 357: 787-794.
[2] Hooper N, Karran E, Turner A. Membrane protein secretases. Biochem. J, 1997, 321: 265-279.
- Posaconazole
Catalog No.:BCC1103
CAS No.:171228-49-2
- N-Methylquipazine dimaleate
Catalog No.:BCC6697
CAS No.:171205-17-7
- PD 158780
Catalog No.:BCC7434
CAS No.:171179-06-9
- H-Ser(tBu)-OMe.HCl
Catalog No.:BCC3033
CAS No.:17114-97-5
- EGLU
Catalog No.:BCC6871
CAS No.:170984-72-2
- Tetrindole mesylate
Catalog No.:BCC6763
CAS No.:170964-68-8
- Dihydroactinidiolide
Catalog No.:BCN6890
CAS No.:17092-92-1
- Donitriptan hydrochloride
Catalog No.:BCC7742
CAS No.:170911-68-9
- Aburatubolactam A
Catalog No.:BCN1821
CAS No.:170894-24-3
- Persianone
Catalog No.:BCN7359
CAS No.:170894-20-9
- Cyasterone
Catalog No.:BCN5416
CAS No.:17086-76-9
- Sonepiprazole
Catalog No.:BCC7879
CAS No.:170858-33-0
- (RS)-AMPA hydrobromide
Catalog No.:BCC6926
CAS No.:171259-81-7
- Delphinidin-3-O-arabinoside chloride
Catalog No.:BCN3021
CAS No.:171370-55-1
- Ethyl 4-hydroxyphenylacetate
Catalog No.:BCN3792
CAS No.:17138-28-2
- Calcium Gluceptate
Catalog No.:BCC3743
CAS No.:17140-60-2
- Otophylloside B 4'''-O-alpha-L-cymaropyranoside
Catalog No.:BCN7511
CAS No.:171422-82-5
- Otophylloside B 4'''-O-beta-D-cymaropyranoside
Catalog No.:BCN7522
CAS No.:171422-85-8
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- Dammarenediol II 3-O-caffeate
Catalog No.:BCN6519
CAS No.:171438-55-4
- Salprionin
Catalog No.:BCN3162
CAS No.:171439-43-3
- Myrislignan
Catalog No.:BCN1242
CAS No.:171485-39-5
- Alexidine dihydrochloride
Catalog No.:BCC2466
CAS No.:1715-30-6
- Urocortin (rat)
Catalog No.:BCC5789
CAS No.:171543-83-2
A high-sensitivity enzyme immunoassay for the quantification of soluble human semaphorin 4D in plasma.[Pubmed:30879960]
Anal Biochem. 2019 Mar 14;574:15-22.
Human semaphorin 4D (SEMA4D), a type I integral membrane glycoprotein, regulates key cellular functions (e.g. cell-cell communication, platelet activation). Its 120kDa extracellular region can be shed from the membrane to release soluble SEMA4D (sSEMA4D). Studies on circulating sSEMA4D levels are mostly performed with poorly characterized assays and use serum and plasma as matrix. We developed and validated a sandwich ELISA utilizing two monoclonal antibodies with resolved epitopes and determined affinities. Human serum and plasma samples were analyzed, and the influence of protease activity on sSEMA4D concentration was tested by collecting samples in the presence of the protease inhibitor TAPI-1. Both antibodies recognize conformational epitopes in the sema domain. Validation for plasma (EDTA, citrate, heparin) showed valid specificity, precision, accuracy, dilution linearity, and robustness. The assay shows a calibration range from 62.5 to 2000pmol/L with a quantification limit of 31pmol/L. sSEMA4D was significantly higher in serum than in plasma, whereas serum and plasma levels from samples collected in the presence of TAPI-1 showed no statistical difference. This ELISA provides a reliable tool for the quantification of sSEMA4D in human plasma. Serum is not recommended as matrix due to the accumulation of shed SEMA4D during blood coagulation altering serum sSEMA4D levels.
EGFR-dependent IL8 Production by Airway Epithelial Cells after Exposure to the Food Flavoring Chemical 2,3-butanedione.[Pubmed:30851105]
Toxicol Sci. 2019 Mar 9. pii: 5372722.
2,3-butanedione (DA), a component of artificial butter flavoring, is associated with the development of occupational bronchiolitis obliterans (BO), a disease of progressive airway fibrosis resulting in lung function decline. Neutrophilic airway inflammation is a consistent feature of BO across a range of clinical contexts and may contribute to disease pathogenesis. Therefore, we sought to determine the importance of the neutrophil chemotactic cytokine interleukin-8 (IL-8) in DA induced lung disease using in-vivo and in-vitro model systems. First, we demonstrated that levels of Cinc-1, the rat homolog of IL-8, are increased in the lung fluid and tissue compartment in a rat model of DA-induced BO. Next, we demonstrated that DA increased IL-8 production by the pulmonary epithelial cell line NCI-H292 and by primary human airway epithelial cells grown under physiologically relevant conditions at an air-liquid interface. We then tested the hypothesis that DA induced epithelial IL-8 protein occurs in an EGFR dependent manner. In these in vitro experiments we demonstrated that epithelial IL-8 protein is blocked by the EGFR tyrosine kinase inhibitor AG1478 and by inhibition of tumor necrosis factor-alpha converting enzyme (TACE) using the small molecule inhibitor, TAPI-1. Finally, we demonstrated that DA induced IL-8 is dependent upon ERK1/2 and MEK activation downstream of EGFR signaling using the small molecule inhibitors AG1478 and PD98059. Together these novel in-vivo and in-vitro observations support that EGFR dependent IL-8 production occurs in DA induced BO. Further studies are warranted to determine the importance of IL-8 in BO pathogenesis.
Effect of soluble cleavage products of important receptors/ligands on efferocytosis: Their role in inflammatory, autoimmune and cardiovascular disease.[Pubmed:30639340]
Ageing Res Rev. 2019 Mar;50:43-57.
Efferocytosis, the clearance of apoptotic cells (ACs), is a physiologic, multifaceted and dynamic process and a fundamental mechanism for the preservation of tissue homeostasis by avoiding unwanted in fl ammation and autoimmune responses through special phagocytic receptors. Defective efferocytosis is associated with several disease states, including cardiovascular disease and impaired immune surveillance, as occurs in cancer and autoimmune disease. A major cause of defective efferocytosis is non-functionality of surface receptors on either the phagocytic cells or the ACs, such as TAM family tyrosine kinase, which turns to a soluble form by cleavage/shedding or alternative splicing. Recently, soluble forms have featured prominently as potential biomarkers, indicative of prognosis and enabling targeted therapy using several commonly employed drugs and inhibitors, such as bleomycin, dexamethasone, statins and some matrix metalloproteinase inhibitors such as TAPI-1 and BB3103. Importantly, to design drug carriers with enhanced circulatory durability, the adaptation of soluble forms of physiological receptors/ligands has been purported. Research has shown that soluble forms are more effective than antibody forms in enabling targeted treatment of certain conditions, such as autoimmune diseases. In this review, we sought to summarize the current knowledge of these soluble products, how they are generated, their interactions, roles, and their potential use as biomarkers in prognosis and treatment related to inflammatory, cardiovascular, and autoimmune diseases.
Resolvin D1, but not resolvin E1, transactivates the epidermal growth factor receptor to increase intracellular calcium and glycoconjugate secretion in rat and human conjunctival goblet cells.[Pubmed:30513286]
Exp Eye Res. 2019 Mar;180:53-62.
PURPOSE: To identify interactions of the epidermal growth factor receptor (EGFR) with the pro-resolving mediator receptors for RvD1 and RvE1 to stimulate an increase in intracellular [Ca(2+)] ([Ca(2+)]i) and mucin secretion from cultured human and rat conjunctival goblet cells. METHODS: Goblet cells from human and rat conjunctiva were grown in culture using RPMI media. Cultured goblet cells were pre-incubated with inhibitors, and then stimulated with RvD1, RvE1, EGF or the cholinergic agonist carbachol (Cch). Increase in [Ca(2+)]i was measured using fura-2/AM. Goblet cell secretion was measured using an enzyme-linked lectin assay with UEA-1. Western blot analysis was performed with antibodies against AKT and ERK 1/2. RESULTS: In cultured human conjunctival goblet cells RvE1 -stimulated an increase in [Ca(2+)]i. RvD1-, but not the RvE1-, stimulated increase in [Ca(2+)]i and mucin secretion was blocked by the EGFR inhibitor AG1478 and siRNA for the EGFR. RvD1-, but not RvE1-stimulated an increase in [Ca(2+)]i that was also inhibited by TAPI-1, an inhibitor of the matrix metalloprotease ADAM 17. Inhibition of the EGFR also blocked RvD1-stimulated increase in AKT activity and both RvD1-and RvE1-stimulated increase in ERK 1/2 activity. Pretreatment with either RvD1 or RvE1 did not block the EGFR-stimulated increase in [Ca(2+)]i. CONCLUSIONS: We conclude that in cultured rat and human conjunctival goblet cells, RvD1 activates the EGFR, increases [Ca(2+)]i, activates AKT and ERK1/2 to stimulate mucin secretion. RvE1 does not transactivate the EGFR to increase [Ca(2+)]I and stimulate mucin secretion, but does interact with the receptor to increase ERK 1/2 activity.
Activation of the EGF Receptor by Histamine Receptor Subtypes Stimulates Mucin Secretion in Conjunctival Goblet Cells.[Pubmed:30025103]
Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3543-3553.
Purpose: The purpose of this study was to determine if histamine receptors interact with the epidermal growth factor receptor (EGFR) in cultured rat conjunctival goblet cells. Methods: Goblet cells from rat conjunctiva were grown in organ culture. First-passage goblet cells were used in all experiments. Phosphorylated (active) and total EGFR, AKT, and extracellular signal-regulated kinase (ERK)1/2 were measured by Western blot analysis. Cells were preincubated with the EGFR antagonist AG1478 for 30 minutes or small interfering RNA specific to the EGFR for 3 days prior to stimulation with histamine or agonists specific for histamine receptor subtypes for 2 hours. Goblet cell secretion was measured using an enzyme-linked lectin assay. Goblet cells were incubated for 1 hour with the calcium indicator molecule fura-2/AM, and intracellular [Ca2+] ([Ca2+]i) was determined. Data were collected in real time and presented as the actual [Ca2+]i with time and as the change in peak [Ca2+]i. Results: Histamine increased the phosphorylation of the EGFR. Mucin secretion and increase in [Ca2+]i stimulated by histamine, and agonists specific for each histamine receptor subtype were blocked by inhibition of the EGFR. Increase in [Ca2+]i stimulated by histamine and specific agonists for each histamine receptor was also inhibited by TAPI-1, a matrix metalloproteinase (MMP) inhibitor. The histamine-stimulated increase in activation of AKT, but not ERK1/2, was blocked by AG1478. Conclusions: In conjunctival goblet cells, histamine, using all four receptor subtypes, transactivates the EGFR via an MMP. This in turn phosphorylates AKT to increase [Ca2+]i and stimulate mucin secretion.
Regenerating hair cells in vestibular sensory epithelia from humans.[Pubmed:30019672]
Elife. 2018 Jul 18;7. pii: 34817.
Human vestibular sensory epithelia in explant culture were incubated in gentamicin to ablate hair cells. Subsequent transduction of supporting cells with ATOH1 using an Ad-2 viral vector resulted in generation of highly significant numbers of cells expressing the hair cell marker protein myosin VIIa. Cells expressing myosin VIIa were also generated after blocking the Notch signalling pathway with TAPI-1 but less efficiently. Transcriptomic analysis following ATOH1 transduction confirmed up-regulation of 335 putative hair cell marker genes, including several downstream targets of ATOH1. Morphological analysis revealed numerous cells bearing dense clusters of microvilli at the apical surfaces which showed some hair cell-like characteristics confirming a degree of conversion of supporting cells. However, no cells bore organised hair bundles and several expected hair cell markers genes were not expressed suggesting incomplete differentiation. Nevertheless, the results show a potential to induce conversion of supporting cells in the vestibular sensory tissues of humans.
EpCAM Intracellular Domain Promotes Porcine Cell Reprogramming by Upregulation of Pluripotent Gene Expression via Beta-catenin Signaling.[Pubmed:28393933]
Sci Rep. 2017 Apr 10;7:46315.
Previous study showed that expression of epithelial cell adhesion molecule (EpCAM) was significantly upregulated in porcine induced pluripotent stem cells (piPSCs). However, the regulatory mechanism and the downstream target genes of EpCAM were not well investigated. In this study, we found that EpCAM was undetectable in fibroblasts, but highly expressed in piPSCs. Promoter of EpCAM was upregulated by zygotic activated factors LIN28, and ESRRB, but repressed by maternal factors OCT4 and SOX2. Knocking down EpCAM by shRNA significantly reduced the pluripotent gene expression. Conversely, overexpression of EpCAM significantly increased the number of alkaline phosphatase positive colonies and elevated the expression of endogenous pluripotent genes. As a key surface-to-nucleus factor, EpCAM releases its intercellular domain (EpICD) by a two-step proteolytic processing sequentially. Blocking the proteolytic processing by inhibitors TAPI-1 and DAPT could reduce the intracellular level of EpICD and lower expressions of OCT4, SOX2, LIN28, and ESRRB. We noticed that increasing intracellular EpICD only was unable to improve activity of EpCAM targeted genes, but by blocking GSK-3 signaling and stabilizing beta-catenin signaling, EpICD could then significantly stimulate the promoter activity. These results showed that EpCAM intracellular domain required beta-catenin signaling to enhance porcine cell reprogramming.
IL-6 trans-signaling is another pathway to upregulate Osteopontin.[Pubmed:27863335]
Cytokine. 2017 Feb;90:88-95.
BACKGROUND: Osteopontin (OPN) is a pro-fibrotic molecule upregulated by pro-inflammatory cytokines. Interleukin (IL)-6 functions downstream of IL-1beta and has unique signal pathways: classic- or trans-signaling via membrane-bound IL-6R or soluble IL-6R (sIL-6R). We investigated the effect of IL-6 trans-signaling on the upregulation of OPN. METHODS: We used THP-1 cells and THP-1 macrophages differentiated from THP-1 cells using phorbol 12-myristate 13-acetate (PMA). After IL-1beta stimulation, expression of OPN, IL-6, sIL-6R, and a disintegrin and metalloproteinase 17 (ADAM17) was examined by ELISA and quantitative PCR. The effects of anti-human IL-6 neutralizing antibody, soluble gp130 (sgp130, IL-6 trans-signaling-specific inhibitor), TAPI-1 (ADAM inhibitor) and siRNA against IL-6R or ADAM17 on OPN expression were evaluated. RESULTS: IL-1beta increased OPN and induced IL-6 in THP-1 macrophages. Anti-IL-6 neutralizing antibody and siRNA against IL-6R inhibited OPN upregulation induced by IL-1beta. TAPI-1 significantly inhibited the increase in sIL-6R induced by IL-1beta. Treatment with sgp130 attenuated OPN elevation by IL-1beta, whereas sgp130 did not change OPN levels in THP-1 macrophages without IL-1beta stimulation. ADAM17 was expressed in THP-1 macrophages and THP-1 cells and IL-1beta stimulation significantly increased ADAM17 expression, regardless of PMA treatment. TAPI-1 and siRNA against ADAM17 significantly inhibited OPN increased by IL-1beta. CONCLUSIONS: IL-6 and sIL-6R induced by IL-1beta may trigger IL-6 trans-signaling, contributing to the upregulation of OPN in THP-1 macrophages. Macrophages may be used as a source of IL-6 and sIL-6R and evoke IL-6 trans-signaling.
Functional Differences of Very-Low-Density Lipoprotein Receptor Splice Variants in Regulating Wnt Signaling.[Pubmed:27528615]
Mol Cell Biol. 2016 Sep 26;36(20):2645-54.
The very-low-density lipoprotein receptor (VLDLR) negatively regulates Wnt signaling. VLDLR has two major alternative splice variants, VLDLRI and VLDLRII, but their biological significance and distinction are unknown. Here we found that most tissues expressed both VLDLRI and VLDLRII, while the retina expressed only VLDLRII. The shed soluble VLDLR extracellular domain (sVLDLR-N) was detected in the conditioned medium of retinal pigment epithelial cells, interphotoreceptor matrix, and mouse plasma, indicating that ectodomain shedding of VLDLR occurs endogenously. VLDLRII displayed a higher ectodomain shedding rate and a more potent inhibitory effect on Wnt signaling than VLDLRI in vitro and in vivo O-glycosylation, which is present in VLDLRI but not VLDLRII, determined the differential ectodomain shedding rates. Moreover, the release of sVLDLR-N was inhibited by a metalloproteinase inhibitor, TAPI-1, while it was promoted by phorbol 12-myristate 13-acetate (PMA). In addition, sVLDLR-N shedding was suppressed under hypoxia. Further, plasma levels of sVLDLR-N were reduced in both type 1 and type 2 diabetic mouse models. We concluded that VLDLRI and VLDLRII had differential roles in regulating Wnt signaling and that decreased plasma levels of sVLDLR-N may contribute to Wnt signaling activation in diabetic complications. Our study reveals a novel mechanism for intercellular regulation of Wnt signaling through VLDLR ectodomain shedding.
Human neutrophil elastase induces MUC5AC overexpression in chronic rhinosinusitis through tumour necrosis factor-alpha converting enzyme.[Pubmed:26881964]
Acta Otolaryngol. 2016 Jun;136(6):641-8.
Conclusion The HNE-TACE signalling pathway has an important role in the process of MUC5AC overexpression in chronic rhinosinusitis (CRS). Objectives To provide evidence of HNE-induced MUC5AC overexpression in CRS via TACE. Method HE and PAS staining were used to assess the pathological changes in sinus mucosa samples from CRS or normal control. HNE, TACE, and MUC5AC expression in the sinonasal mucosa was determined using immunohistochemistry (IHC) and real-time polymerase chain reaction (qRT-PCR). In addition, the MUC5AC and TACE expression was determined in a primary culture of human nasal mucosa epithelial cells in vitro. Results On HE staining, the main pathological feature in the sinus mucosa of CRS patients was hyperplasia of goblet cells, inflammatory cells, and submucosal glands. Mucosa from the two experimental groups also showed strong expression on PAS staining. IHC and qRT-PCR demonstrated that HNE, TACE, and MUC5AC expression was significantly higher in the CRS patients compared with control samples (p < 0.05). MUC5AC mRNA expression was higher in cells stimulated by HNE than in untreated cells (p < 0.05). MUC5AC mRNA expression was significantly reduced in cells pre-treated with the TACE inhibitor TAPI-1 prior to HNE stimulation, compared with untreated and HNE-stimulated cells (p < 0.01).
The activation of EGFR promotes myocardial tumor necrosis factor-alpha production and cardiac failure in endotoxemia.[Pubmed:26486084]
Oncotarget. 2015 Nov 3;6(34):35478-95.
To study the effect of EGFR activation on the generation of TNF-alpha and the occurrence of cardiac dysfuncetion during sepsis, PD168393 and erlotinib (both are EGFR inhibitors) were applied to decreased the production of TNF-alpha and phosphrylation of ERK1/2 and p38 induced by LPS in cardiomyocytes. These results were further proved by specifically knocked down the expression of EGFR in vitro. Both TAPI-1, a TNF-alpha converting enzyme (TACE) inhibitor, and TGF-alpha neutralizing antibody could inhibit the activation of EGFR and the generation of TNF-alpha mRNA after LPS treatment. The increase of TGF-alpha in response to LPS could also be suppressed by TAPI-1. On the other hand, exogenous TGF-alpha increased the expression of TNF-alpha mRNA and partially reversed the inhibitory effect of TAPI-1 on expression of TNF-alpha mRNA in response to LPS indicating that the transactivation of EGFR by LPS in cardiomyocytes needs the help of TACE and TGF-alpha. In endotoxemic mice, inhibition the activation of EGFR not only decreased TNF-alpha production in the myocardium but also improved left ventricular pump function and ameliorated cardiac dysfunction and ultimately improved survival rate. All these results provided a new insight of how EGFR regulation the production of TNF-alpha in cardiomyocytes and a potential new target for the treatment of cardiac dysfunction in sepsis.
[Effects of tumor necrosis factor-alpha converting enzyme on mucous hypersecretion in inflammatory airway].[Pubmed:25544160]
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014 Dec;39(12):1228-32.
OBJECTIVE: To investigate the effect of tumor necrosis factor-alpha converting enzyme (TACE) on mucous hypersecretion in inflammatory airway. METHODS: Mucous hypersecretion model of human lung adenocarcinoma cells A549 was induced by human neutrophil elastase (HNE), and TNF-alpha converting enzyme inhibitor-1 (TAPI-1), an inhibitor of TACE, was chosen for the inference study. The expression of MUC5AC and TACE was examined. Th e cells were divided into 5 groups: a negative control group, HNE1 (15 nmol/L) group, HNE2 (25 nmol/L) group, HNE3 (50 nmol/L) group and TAPI-1 group. RT-PCR was used to examine MUC5AC and TACE mRNA expression. The protein expression of TACE and MUC5AC was examined by Western blot and ELISA, respectively. RESULTS: HNE induced the TACE and MUC5AC mRNA and protein expression in a dose-dependent manner. Compared with the control group, the increases were all significantly increased in the three dosages of HNE group (P< 0.01). The HNE-induced TACE and MUC5AC mRNA and protein expression were dramatically attenuated in the presence of TAPI-1, an inhibitor of TACE (P< 0.01). CONCLUSION: TACE participated cell signalling pathway of airway mucous hypersecretion, and could down regulation the level of inflammation airway mucous hypersecretion.


