N-Methyltaxol CCAS# 153083-53-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 153083-53-5 | SDF | Download SDF |
| PubChem ID | 10260022 | Appearance | Powder |
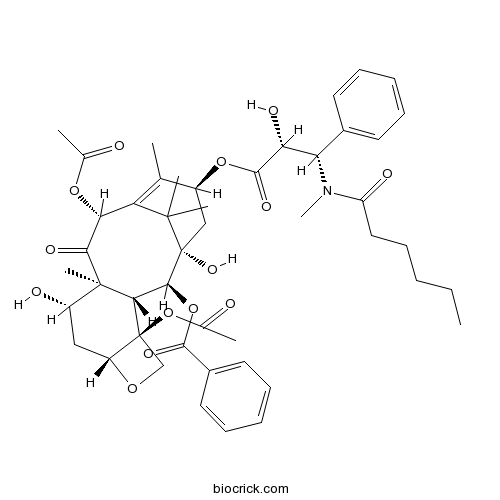
| Formula | C47H59NO14 | M.Wt | 861.97 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CCCCCC(=O)N(C)C(C1=CC=CC=C1)C(C(=O)OC2CC3(C(C4C(C(CC5C4(CO5)OC(=O)C)O)(C(=O)C(C(=C2C)C3(C)C)OC(=O)C)C)OC(=O)C6=CC=CC=C6)O)O | ||
| Standard InChIKey | LJTMOSWWGSCCPR-AMMYIWEDSA-N | ||
| Standard InChI | InChI=1S/C47H59NO14/c1-9-10-13-22-34(52)48(8)36(29-18-14-11-15-19-29)37(53)43(56)60-31-24-47(57)41(61-42(55)30-20-16-12-17-21-30)39-45(7,32(51)23-33-46(39,25-58-33)62-28(4)50)40(54)38(59-27(3)49)35(26(31)2)44(47,5)6/h11-12,14-21,31-33,36-39,41,51,53,57H,9-10,13,22-25H2,1-8H3/t31-,32-,33+,36-,37+,38+,39-,41-,45+,46-,47+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. N-methyltaxol C and paclitaxel can induced conduction arrhythmias and reduce coronary flow and left ventricular systolic pressure in the isolated heart. 2. N-methyltaxol C and paclitaxel can produce a positive inotropic effect in papillary muscle, without alterations in the action potential. |

N-Methyltaxol C Dilution Calculator

N-Methyltaxol C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1601 mL | 5.8007 mL | 11.6013 mL | 23.2027 mL | 29.0033 mL |
| 5 mM | 0.232 mL | 1.1601 mL | 2.3203 mL | 4.6405 mL | 5.8007 mL |
| 10 mM | 0.116 mL | 0.5801 mL | 1.1601 mL | 2.3203 mL | 2.9003 mL |
| 50 mM | 0.0232 mL | 0.116 mL | 0.232 mL | 0.4641 mL | 0.5801 mL |
| 100 mM | 0.0116 mL | 0.058 mL | 0.116 mL | 0.232 mL | 0.29 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Diclofenac
Catalog No.:BCC5249
CAS No.:15307-86-5
- Diclofenac Sodium
Catalog No.:BCC4439
CAS No.:15307-79-6
- SR 140333
Catalog No.:BCC6098
CAS No.:153050-21-6
- Precursor of cefcapene diisopropylanmine salt
Catalog No.:BCC9127
CAS No.:153012-37-4
- Serotonin HCl
Catalog No.:BCC4715
CAS No.:153-98-0
- H-D-Trp-OH
Catalog No.:BCC3117
CAS No.:153-94-6
- 2-Aminofluorene
Catalog No.:BCC8549
CAS No.:153-78-6
- Rutin
Catalog No.:BCN1684
CAS No.:153-18-4
- Guajadial F
Catalog No.:BCN6437
CAS No.:1529775-08-3
- Guajadial E
Catalog No.:BCN7754
CAS No.:1529775-06-1
- Guajadial D
Catalog No.:BCN7756
CAS No.:1529775-04-9
- Guajadial C
Catalog No.:BCN7755
CAS No.:1529775-02-7
- Thiamphenicol
Catalog No.:BCC4736
CAS No.:15318-45-3
- Taxayunnansin A
Catalog No.:BCN1685
CAS No.:153229-31-3
- Cilomilast
Catalog No.:BCC2283
CAS No.:153259-65-5
- ML355
Catalog No.:BCC8060
CAS No.:1532593-30-8
- 4,4'-Bis(2-benzoxazolyl)stilbene
Catalog No.:BCC8656
CAS No.:1533-45-5
- DFB
Catalog No.:BCC7130
CAS No.:15332-10-2
- SCR7
Catalog No.:BCC3978
CAS No.:1533426-72-0
- Ginkgolide K
Catalog No.:BCN8209
CAS No.:153355-70-5
- 7-Epi-docetaxel
Catalog No.:BCC5411
CAS No.:153381-68-1
- Taxol C
Catalog No.:BCN6941
CAS No.:153415-45-3
- Taxcultine
Catalog No.:BCN6948
CAS No.:153415-46-4
- Gavestinel
Catalog No.:BCC7340
CAS No.:153436-38-5
Differential effects of paclitaxel and derivatives on guinea pig isolated heart and papillary muscle.[Pubmed:9454798]
J Pharmacol Exp Ther. 1998 Feb;284(2):561-7.
Paclitaxel (Taxol) is an anticancer agent with clinical activity against various human cancer types. Paclitaxel blocks cell division by stabilizing microtubules, a mechanism that also underlies its major side effects (neutropenia and neurotoxicity). Paclitaxel can also alter cardiac function, and to elucidate the mechanism of this activity, we tested the mechanical and electrical effects of paclitaxel and a series of analogs (docetaxel, taxol B, taxol C and N-Methyltaxol C; 5-20 microM) on two different cardiac preparations, the isolated coronary perfused heart and the papillary muscle of the guinea pig. Paclitaxel and N-Methyltaxol C induced conduction arrhythmias and reduced coronary flow and left ventricular systolic pressure in the isolated heart, whereas the other taxol derivatives tested had no significant effect. Moreover, paclitaxel blocked the vasodilator effect of bradykinin in the isolated heart. Paclitaxel and N-Methyltaxol C produced a positive inotropic effect in papillary muscle, without alterations in the action potential. In the latter preparation, no significant variations were observed after treatment with the other taxol derivatives. The in vitro cardiodepressant and arrhythmogenic activity of paclitaxel is similar to that reported after its clinical administration and might be due to coronary vasoconstriction. The precise role of microtubules as modulators of intracellular calcium in cardiac and smooth muscle cells is at present unclear, because docetaxel and other taxol analogs, though they exhibited similar activity on tubulin, lacked cardiac effects.
N-methylation of the c3' amide of taxanes: synthesis of N-methyltaxol C and N-methylpaclitaxel.[Pubmed:18498195]
J Org Chem. 2008 Jun 20;73(12):4705-8.
A method has been developed for the methylation of the C3' amide of taxol C and paclitaxel. Taxol C and paclitaxel were sequentially silylated at the 2', 7, and 1-hydroxyl groups with tert-butyldimethylsilyl chloride, triethylsilyl chloride, and dimethylsilyl chloride, respectively. Subsequent reaction with potassium tert-butoxide and methyl iodide provided the corresponding N-methylated taxane derivatives. Removal of the silyl protecting groups furnished N-Methyltaxol C and N-methylpaclitaxel.


