Neoprzewaquinone ACAS# 630057-39-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 630057-39-5 | SDF | Download SDF |
| PubChem ID | 124222343 | Appearance | Red powder |
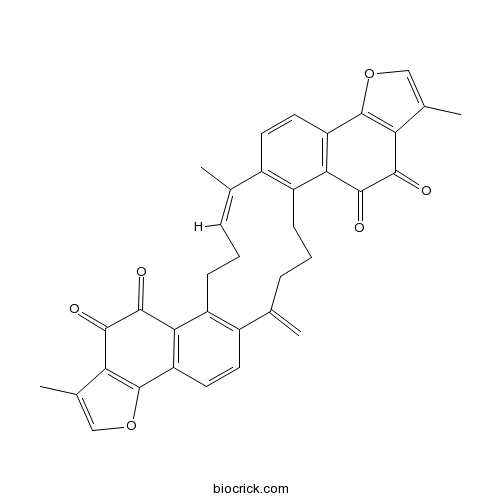
| Formula | C36H28O6 | M.Wt | 556.6 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2Z)-2,11,28-trimethyl-19-methylidene-13,30-dioxaheptacyclo[21.11.0.06,18.07,15.010,14.024,32.027,31]tetratriaconta-1(23),2,6(18),7(15),10(14),11,16,24(32),27(31),28,33-undecaene-8,9,25,26-tetrone | ||
| SMILES | CC1=CCCC2=C(C=CC3=C2C(=O)C(=O)C4=C3OC=C4C)C(=C)CCCC5=C1C=CC6=C5C(=O)C(=O)C7=C6OC=C7C | ||
| Standard InChIKey | SXQCYGZVSVUMEL-LSCVHKIXSA-N | ||
| Standard InChI | InChI=1S/C36H28O6/c1-17-7-5-9-24-22(12-14-26-30(24)34(40)32(38)28-20(4)16-42-36(26)28)18(2)8-6-10-23-21(17)11-13-25-29(23)33(39)31(37)27-19(3)15-41-35(25)27/h8,11-16H,1,5-7,9-10H2,2-4H3/b18-8- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Neoprzewaquinone A has algicidal effect on M. aeruginosa , with EC50 of 4.68 mg /L, the potential inhibition mechanisms are neo-przewaquinone A caused M. aeruginosa cells morphologic damage or lysis. |
| Targets | Antifection |
| In vitro | Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa--towards identification of algicidal substance and determination of inhibition mechanism.[Pubmed: 23810520]Chemosphere. 2013 Oct;93(6):997-1004.The present study was to isolate and identify a potent algicidal compound from extract of Salvia miltiorrhiza and study the potential inhibition mechanism on Microcystis aeruginosa.
|
| Structure Identification | Yao Xue Xue Bao. 2003 May;38(5):354-7.Chemical constituents in the roots of Salvia przewalskii Maxim.[Pubmed: 12958839]To investigate the chemical composition of the root of Salvia przewalskii Maxim.
|

Neoprzewaquinone A Dilution Calculator

Neoprzewaquinone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7966 mL | 8.9831 mL | 17.9662 mL | 35.9324 mL | 44.9156 mL |
| 5 mM | 0.3593 mL | 1.7966 mL | 3.5932 mL | 7.1865 mL | 8.9831 mL |
| 10 mM | 0.1797 mL | 0.8983 mL | 1.7966 mL | 3.5932 mL | 4.4916 mL |
| 50 mM | 0.0359 mL | 0.1797 mL | 0.3593 mL | 0.7186 mL | 0.8983 mL |
| 100 mM | 0.018 mL | 0.0898 mL | 0.1797 mL | 0.3593 mL | 0.4492 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Corynoxeine
Catalog No.:BCN5002
CAS No.:630-94-4
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
- Ouabain
Catalog No.:BCC5069
CAS No.:630-60-4
- Nonacosane
Catalog No.:BCC9102
CAS No.:630-03-5
- Phenoxybenzamine HCl
Catalog No.:BCC4334
CAS No.:63-92-3
- L-Phenylalanine
Catalog No.:BCN3818
CAS No.:63-91-2
- Sulfanilamide
Catalog No.:BCC4858
CAS No.:63-74-1
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Primaquine Diphosphate
Catalog No.:BCC4706
CAS No.:63-45-6
- Androstenedione
Catalog No.:BCC8296
CAS No.:63-05-8
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
- 6-Aminoquinoxaline
Catalog No.:BCC8767
CAS No.:6298-37-9
- MRS 2500 tetraammonium salt
Catalog No.:BCC5881
CAS No.:630103-23-0
- PD 168077 maleate
Catalog No.:BCC6919
CAS No.:630117-19-0
- AST 487
Catalog No.:BCC1373
CAS No.:630124-46-8
- Androstenone hydrazone
Catalog No.:BCC8830
CAS No.:63015-10-1
- Crenulatin
Catalog No.:BCN7791
CAS No.:63026-02-8
- Hexacosyl (E)-ferulate
Catalog No.:BCN4170
CAS No.:63034-29-7
- Senkyunolide
Catalog No.:BCN8154
CAS No.:63038-10-8
- H-Tle-OMe.HCl
Catalog No.:BCC2658
CAS No.:63038-27-7
- Estradiol-3-benzoate-17-butyrate
Catalog No.:BCC8963
CAS No.:63042-18-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- H-Val-OMe.HCl
Catalog No.:BCC3142
CAS No.:6306-52-1
- Boc-Thr-OSu
Catalog No.:BCC3450
CAS No.:63076-44-8
[Chemical constituents in the roots of Salvia przewalskii Maxim].[Pubmed:12958839]
Yao Xue Xue Bao. 2003 May;38(5):354-7.
AIM: To investigate the chemical composition of the root of Salvia przewalskii Maxim. METHODS: Compounds were isolated by silica gel column chromatography. Structures of these compounds were elucidated by spectral analysis (EI-MS, FAB-MS, 1HNMR, 13CNMR, 1H-1H COSY, 1H-13C COSY, HMBC, NOESY) and phytochemical properties. RESULTS: Eight compounds were isolated and identified as: tanshinone II-A (I), crypotanshinone (II), przewaquinone A (III), sugiol (IV), ursolic acid (V), 2 alpha, 3 alpha-dihydroxy urs-12-ene-28-acid (VI), oleanolic acid (VII), and neo-przewaquinone A (VIII). CONCLUSION: Compound VIII is a new compound, and compound II, IV, V, VI and VII are isolated from this plant for the first time.
Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa--towards identification of algicidal substance and determination of inhibition mechanism.[Pubmed:23810520]
Chemosphere. 2013 Oct;93(6):997-1004.
The present study was to isolate and identify a potent algicidal compound from extract of Salvia miltiorrhiza and study the potential inhibition mechanism on Microcystis aeruginosa. Column chromatography and bioassay-guided fractionation methods were carried out to yield neo-przewaquinone A, which was identified by spectral analysis. The EC50 of neo-przewaquinone A on M. aeruginosa were 4.68 mg L(-1). In addition, neo-przewaquinone A showed relatively higher security on Chlorella pyrenoidosa and Scenedesmus obliquus, with the EC50 values of 14.78 and 10.37 mg L(-1), respectively. For the potential inhibition mechanisms, neo-przewaquinone A caused M. aeruginosa cells morphologic damage or lysis, increased malondialdehyde content and decreased the soluble protein content, total antioxidant and superoxide dismutase activity, and significantly inhibited three photosynthesis-related genes (psaB, psbD, and rbcL). The results demonstrated the algicidal effect of neo-przewaquinone A on M. aeruginosa and provided the possible inhibition mechanisms.


