AST 487RET kinase inhibitor CAS# 630124-46-8 |
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
- Quizartinib (AC220)
Catalog No.:BCC2548
CAS No.:950769-58-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 630124-46-8 | SDF | Download SDF |
| PubChem ID | 11409972 | Appearance | Powder |
| Formula | C26H30F3N7O2 | M.Wt | 529.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NVP-AST 487 | ||
| Solubility | >26.5mg/mL in DMSO | ||
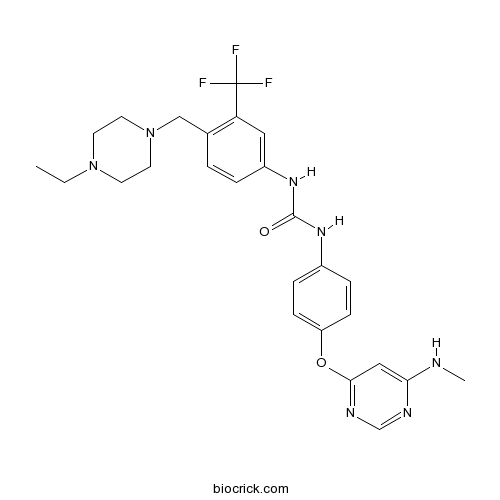
| Chemical Name | 1-[4-[(4-ethylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]-3-[4-[6-(methylamino)pyrimidin-4-yl]oxyphenyl]urea | ||
| SMILES | CCN1CCN(CC1)CC2=C(C=C(C=C2)NC(=O)NC3=CC=C(C=C3)OC4=NC=NC(=C4)NC)C(F)(F)F | ||
| Standard InChIKey | ODPGGGTTYSGTGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AST487 is an inhibitor of RET kinase with IC50 value of 0.88 μM. | |||||
| Targets | RET kinase | |||||
| IC50 | 0.88 μM | |||||
| Cell experiment [1]: | |
| Cell lines | Baf3 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 10 min; IC50=34±4 nmol/L |
| Applications | Data derived from a panel of Baf3 murine pro–B cell lymphoma lines rendered growth factor–independent by transduction with various activated tyrosine kinases, suggested cellular specificity for RET-driven proliferation (IC50 for PTC3-RET–driven Baf3 cells, 34±4 nmol/L), with activity against FLT3 as well, and to a lesser extent,Bcr-ABL–dependent proliferation |
| Animal experiment [1]: | |
| Animal models | Female athymic nude mice |
| Dosage form | 50 mg/kg; oral taken |
| Application | NVP-AST487 given p.o. evoked a dose-dependent inhibition of growth of NIH3T3-RETC634W xenografts, with doses >30 mg/kg/d causing significant reductions in tumor size. The effects of the compound on RET expression and phosphorylation in tumor extracts was analyzed 6 h following the final treatment. Reductions in tumor RET phosphorylation in NVP-AST487–treated animals were clearly seen, particularly at doses ≥30 mg/kg. Interestingly, there was also a dose-dependent decrease of RET expression, with one of three tumors analyzed in the 30 mg/kg group and three of three tumors in the 50 mg/kg group showing a dramatic reduction in RET protein levels. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Yu K, Toral-Barza L, Shi C, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin[J]. Cancer research, 2009, 69(15): 6232-6240. | |

AST 487 Dilution Calculator

AST 487 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8884 mL | 9.4418 mL | 18.8836 mL | 37.7672 mL | 47.209 mL |
| 5 mM | 0.3777 mL | 1.8884 mL | 3.7767 mL | 7.5534 mL | 9.4418 mL |
| 10 mM | 0.1888 mL | 0.9442 mL | 1.8884 mL | 3.7767 mL | 4.7209 mL |
| 50 mM | 0.0378 mL | 0.1888 mL | 0.3777 mL | 0.7553 mL | 0.9442 mL |
| 100 mM | 0.0189 mL | 0.0944 mL | 0.1888 mL | 0.3777 mL | 0.4721 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AST487 is an inhibitor of RET kinase with IC50 value of 0.88μM [1].
AST487 belongs to the N,N’-diphenyl urea class. It inhibit the activity of RET kinase as well as many other kinases( such as KDR, Flt-3 and c-Kit) in vitro. In the cell assay, the inhibition effect of AST487 is displayed both in PC-RET/PTC3 cells and TT cells, which harbor an endogenous activating point mutation of RET (RETC634W). AST487 decreases RET autophosphorylation and activation of PLCγ and ERK with a dose-dependent manner. Additionally, AST487 is also found to inhibit the growth of human thyroid cancer cell lines with RET, but not BRAF mutations. It supports the selectivity of AST487 for RET. In vivo assay shows that AST487 causes significant reductions in the size of NIH3T3-RETC634W xenografts with doses >30 mg/kg/d and oral administration of AST487 at 50 or 30 mg/kg/d decreases mean tumor volume in mice [1].
References:
[1] Nagako Akeno-Stuart, Michelle Croyle, Jeffrey A. Knauf, et al. The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Res. 2007, 67:6956-6964.
- PD 168077 maleate
Catalog No.:BCC6919
CAS No.:630117-19-0
- MRS 2500 tetraammonium salt
Catalog No.:BCC5881
CAS No.:630103-23-0
- Neoprzewaquinone A
Catalog No.:BCN4169
CAS No.:630057-39-5
- Corynoxeine
Catalog No.:BCN5002
CAS No.:630-94-4
- Phenytoin sodium
Catalog No.:BCC5071
CAS No.:630-93-3
- Ouabain
Catalog No.:BCC5069
CAS No.:630-60-4
- Nonacosane
Catalog No.:BCC9102
CAS No.:630-03-5
- Phenoxybenzamine HCl
Catalog No.:BCC4334
CAS No.:63-92-3
- L-Phenylalanine
Catalog No.:BCN3818
CAS No.:63-91-2
- Sulfanilamide
Catalog No.:BCC4858
CAS No.:63-74-1
- H-Met-OH
Catalog No.:BCC2993
CAS No.:63-68-3
- Primaquine Diphosphate
Catalog No.:BCC4706
CAS No.:63-45-6
- Androstenone hydrazone
Catalog No.:BCC8830
CAS No.:63015-10-1
- Crenulatin
Catalog No.:BCN7791
CAS No.:63026-02-8
- Hexacosyl (E)-ferulate
Catalog No.:BCN4170
CAS No.:63034-29-7
- Senkyunolide
Catalog No.:BCN8154
CAS No.:63038-10-8
- H-Tle-OMe.HCl
Catalog No.:BCC2658
CAS No.:63038-27-7
- Estradiol-3-benzoate-17-butyrate
Catalog No.:BCC8963
CAS No.:63042-18-2
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- H-Val-OMe.HCl
Catalog No.:BCC3142
CAS No.:6306-52-1
- Boc-Thr-OSu
Catalog No.:BCC3450
CAS No.:63076-44-8
- (±)-threo-3-Methylglutamic acid
Catalog No.:BCC6804
CAS No.:63088-04-0
- Quillaic acid
Catalog No.:BCC5310
CAS No.:631-01-6
- Beta-boswellic acid
Catalog No.:BCN2367
CAS No.:631-69-6
Forbidden Coherence Transfer of (19)F Nuclei to Quantitatively Measure the Dynamics of a CF(3)-Containing Ligand in Receptor-Bound States.[Pubmed:28880244]
Molecules. 2017 Sep 7;22(9). pii: molecules22091492.
The dynamic property of a ligand in the receptor-bound state is an important metric to characterize the interactions in the ligand-receptor interface, and the development of an experimental strategy to quantify the amplitude of motions in the bound state is of importance to introduce the dynamic aspect into structure-guided drug development (SGDD). Fluorine modifications are frequently introduced at the hit-to-lead optimization stage to enhance the binding potency and other characteristics of a ligand. However, the effects of fluorine modifications are generally difficult to predict, owing to the pleiotropic nature of the interactions. In this study, we report an NMR-based approach to experimentally evaluate the local dynamics of trifluoromethyl (CF(3))-containing ligands in the receptor-bound states. For this purpose, the forbidden coherence transfer (FCT) analysis, which has been used to study the dynamics of methyl moieties in proteins, was extended to the (19)F nuclei of CF(3)-containing ligands. By applying this CF(3)-FCT analysis to a model interaction system consisting of a ligand, AST-487, and a receptor, p38alpha, we successfully quantified the amplitude of the CF(3) dynamics in the p38alpha-bound state. The strategy would bring the CF(3)-containing ligands within the scope of dynamic SGDD to improve the affinity and specificity for the drug-target receptors.
Saikosaponins induced hepatotoxicity in mice via lipid metabolism dysregulation and oxidative stress: a proteomic study.[Pubmed:28420359]
BMC Complement Altern Med. 2017 Apr 19;17(1):219.
BACKGROUND: Radix Bupleuri (RB) has been popularly used for treating many liver diseases such as chronic hepatic inflammation and viral Hepatitis in China. Increasing clinical and experimental evidence indicates the potential hepatotoxicity of RB or prescriptions containing RB. Recently, Saikosaponins (SS) have been identified as major bioactive compounds isolated from RB, which may be also responsible for RB-induced liver injury. METHODS: Serum AST, ALT and LDH levels were determined to evaluate SS-induced liver injury in mice. Serum and liver total triglyceride and cholesterol were used to indicate lipid metabolism homeostasis. Liver ROS, GSH, MDA and iNOS were used to examine the oxidative stress level after SS administration. Western blot was used to detect CYP2E1 expression. A 8-Plex iTRAQ Labeling Coupled with 2D LC - MS/MS technique was applied to analyze the protein expression profiles in livers of mice administered with different doses of SS for different time periods. Gene ontology analysis, cluster and enrichment analysis were employed to elucidate potential mechanism involved. HepG2 cells were used to identify our findings in vitro. RESULTS: SS dose- and time-dependently induced liver injury in mice, indicated by increased serum AST, ALT and LDH levels. According to proteomic analysis, 487 differentially expressed proteins were identified in mice administrated with different dose of SS for different time periods. Altered proteins were enriched in pathways such as lipid metabolism, protein metabolism, macro molecular transportation, cytoskeleton structure and response to stress. SS enhanced CYP2E1 expression in a time and dose dependent manner, and induced oxidative stress both in vivo and in vitro. CONCLUSION: Our results identified hepatotoxicity and established dose-time course-liver toxicity relationship in mice model of SS administration and suggested potential mechanisms, including impaired lipid and protein metabolism and oxidative stress. The current study provides experimental evidence for clinical safe use of RB, and also new insights into understanding the mechanism by which SS and RB induced liver injury.
Acute temozolomide induced liver injury: Mixed type hepatocellular and cholestatic toxicity.[Pubmed:28209108]
Acta Gastroenterol Belg. 2016 Sep-Dec;79(4):487-489.
Temozolomide (TMZ) is an oral imidazotetrazine methylating agent which is used for the treatment of glioblastoma multiforme (GBM). We report a case of acute hepatotoxicity in a 53-year old male patient after administration of TMZ for GBM. He had fatigue, nausea, anorexia and jaundice. His laboratory analysis showed alanine aminotransferase(ALT): 632 IU/L (normal range 0-40); aspartate aminotransferase(AST): 554 IU/L (normal range 5-34); alkaline phosphatase(ALP): 1143 IU/L (normal range 40-150); gamma-glutamyl transpeptidase(GGT): 514 IU/L (normal range 9-64 IU/L); total bilirubin: 15.1 mg/dL (normal range 0-1.2); direct bilirubin: 13.2 mg/dL and prothrombin time(PT): 13.5 s, with international normalized ratio (INR): 1.1 (normal range 0.8-1.2). His liver biopsy specimen showed mixed-type (both hepatocellular and cholestatic) hepatic injury, compatible with a diagnosis of drug-induced hepatitis. An objective causality assessment using the Naranjo probability scale suggested that TMZ was the probable cause of the acute hepatitis. His liver function tests gradually normalized in 6 months after discontinuation of the drug. In susceptible individuals, TMZ use may lead to acute mixed type liver toxicity. Complete recovery may be possible if the drug is discontinued before severe liver injury is established. (Acta gastroenterol. belg., 2016, 79, 487-489).
[Correlation Between Serological Indices and Liver Histological Pathology in Patients with HBV Infection].[Pubmed:26480673]
Sichuan Da Xue Xue Bao Yi Xue Ban. 2015 Jul;46(4):641-4.
OBJECTIVE: To analyze the correlation between serological indices and liver histological pathology in the patients with hepatitis B virus ( HBV) infection. METHODS: This study enrolled 301 patients with HBV infection. All of them received liver biopsy, serum biochemical examination, including alanineamino transferase (ALT), aspart ateaminotransferase (AST), albumin (ALB), globulin (GLB ), HBV DNA test and HBV genotype detection. RESULTS: ALT, AST and AST/ALT in G2/G(3+4) group were significantly higher than those in group G0/G1 (P < 0.05), and all showed positive correlation with liver inflammation (r = 0.487, 0.648, 0.509, P < 0.05). GLB in S2/S(3+4) group was significantly higher than that in group S0/S1 (P < 0.05), which had positive correlation with liver fibrosis (r = 0.674, P < 0.05). ALB/GLB (A/G) in S2/S(3+4) group was significantly lower than that in group S0/S1 (P < 0.05), it had negative correlation with liver fibrosis(r = -0.500, P < 0.05). The inflammation and fibrosis level in patients with C genotype was higher than that of B genotype (chi2 = 11.460, 12.729, P < 0.05). CONCLUSION: ALT, AST and AST/ALT show better diagnostic value in liver inflammation. GLB and A/G show better diagnostic value in liver fibrosis. The progress of this disease is relatively faster in the patients with C genotype HBV infection.


