OGT 2115Heparanase inhibitor. Antiangiogenic CAS# 853929-59-6 |
- Olprinone
Catalog No.:BCC1820
CAS No.:106730-54-5
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 853929-59-6 | SDF | Download SDF |
| PubChem ID | 44402523 | Appearance | Powder |
| Formula | C24H16BrFN2O4 | M.Wt | 495.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
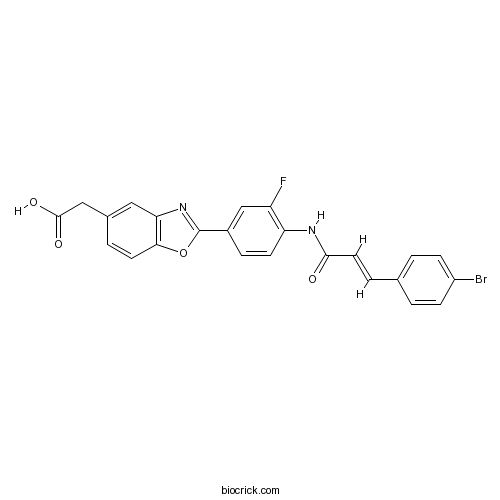
| Chemical Name | 2-[2-[4-[[(E)-3-(4-bromophenyl)prop-2-enoyl]amino]-3-fluorophenyl]-1,3-benzoxazol-5-yl]acetic acid | ||
| SMILES | C1=CC(=CC=C1C=CC(=O)NC2=C(C=C(C=C2)C3=NC4=C(O3)C=CC(=C4)CC(=O)O)F)Br | ||
| Standard InChIKey | LKBXWNYXDMSFQU-ONNFQVAWSA-N | ||
| Standard InChI | InChI=1S/C24H16BrFN2O4/c25-17-6-1-14(2-7-17)4-10-22(29)27-19-8-5-16(13-18(19)26)24-28-20-11-15(12-23(30)31)3-9-21(20)32-24/h1-11,13H,12H2,(H,27,29)(H,30,31)/b10-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Heparanase inhibitor (IC50 = 0.4 μM) that displays no major inhibition of human cytochrome P450 isoenzymes (IC50 > 30 μM). Exhibits antiangiogenic properties in vitro (IC50 = 1 μM). |

OGT 2115 Dilution Calculator

OGT 2115 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.019 mL | 10.0949 mL | 20.1898 mL | 40.3796 mL | 50.4745 mL |
| 5 mM | 0.4038 mL | 2.019 mL | 4.038 mL | 8.0759 mL | 10.0949 mL |
| 10 mM | 0.2019 mL | 1.0095 mL | 2.019 mL | 4.038 mL | 5.0474 mL |
| 50 mM | 0.0404 mL | 0.2019 mL | 0.4038 mL | 0.8076 mL | 1.0095 mL |
| 100 mM | 0.0202 mL | 0.1009 mL | 0.2019 mL | 0.4038 mL | 0.5047 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NVP-BAG956
Catalog No.:BCC1813
CAS No.:853910-02-8
- (+)-AJ 76 hydrochloride
Catalog No.:BCC6747
CAS No.:85378-82-1
- SKF 89976A hydrochloride
Catalog No.:BCC6930
CAS No.:85375-15-1
- 5,19-Epoxy-19R,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1328
CAS No.:85372-72-1
- 5,19-Epoxy-19S,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1329
CAS No.:85372-70-9
- SecinH3
Catalog No.:BCC7503
CAS No.:853625-60-2
- K03861
Catalog No.:BCC6537
CAS No.:853299-07-7
- Trichorabdal A
Catalog No.:BCN4404
CAS No.:85329-59-5
- Hythiemoside A
Catalog No.:BCN4403
CAS No.:853267-91-1
- Ajugalide D
Catalog No.:BCN3665
CAS No.:853247-65-1
- Ajugalide C
Catalog No.:BCN8015
CAS No.:853247-64-0
- PG 01
Catalog No.:BCC7820
CAS No.:853138-65-5
- Dasatinib hydrochloride
Catalog No.:BCC1517
CAS No.:854001-07-3
- Norcaesalpinin E
Catalog No.:BCN7006
CAS No.:854038-96-3
- (-)-Haplomyrfolin
Catalog No.:BCN3225
CAS No.:85404-48-4
- Ropivacaine mesylate
Catalog No.:BCC9137
CAS No.:854056-07-8
- Rilmenidine Phosphate
Catalog No.:BCC5637
CAS No.:85409-38-7
- S- (+)-Rolipram
Catalog No.:BCC2303
CAS No.:85416-73-5
- (R)-(-)-Rolipram
Catalog No.:BCC5429
CAS No.:85416-75-7
- Caffeic anhydride
Catalog No.:BCN3295
CAS No.:854237-32-4
- Ajugamarin chlorohydrin
Catalog No.:BCN3664
CAS No.:85447-27-4
- 4-Chlorotestosterone acetate
Catalog No.:BCC8705
CAS No.:855-19-6
- Eupatorin
Catalog No.:BCN4405
CAS No.:855-96-9
- PK 11195
Catalog No.:BCC6745
CAS No.:85532-75-8
Host Enzymes Heparanase and Cathepsin L Promote Herpes Simplex Virus 2 Release from Cells.[Pubmed:30232188]
J Virol. 2018 Nov 12;92(23). pii: JVI.01179-18.
Herpes simplex virus 2 (HSV-2) can productively infect many different cell types of human and nonhuman origin. Here we demonstrate interconnected roles for two host enzymes, heparanase (HPSE) and cathepsin L, in HSV-2 release from cells. In vaginal epithelial cells, HSV-2 causes heparan sulfate shedding and upregulation in HPSE levels during the productive phase of infection. We also noted increased levels of cathepsin L and show that regulation of HPSE by cathepsin L via cleavage of HPSE proenzyme is important for infection. Furthermore, inhibition of HPSE by a specific inhibitor, OGT 2115, dramatically reduces HSV-2 release from vaginal epithelial cells. Likewise, we show evidence that the inhibition of cathepsin L is detrimental to the infection. The HPSE increase after infection is mediated by an increased NF-kappaB nuclear localization and a resultant activation of HPSE transcription. Together these mechanisms contribute to the removal of heparan sulfate from the cell surface and thus facilitate virus release from cells.IMPORTANCE Genital infections by HSV-2 represent one of the most common sexually transmitted viral infections. The virus causes painful lesions and sores around the genitals or rectum. Intermittent release of the virus from infected tissues during sexual activities is the most common cause of transmission. At the molecular level, cell surface heparan sulfate (HS) is known to provide attachment sites for HSV-2. While the removal of HS during HSV-1 release has been shown, not much is known about the host factors and their regulators that contribute to HSV-2 release from natural target cell types. Here we suggest a role for the host enzyme heparanase in HSV-2 release. Our work reveals that in addition to the regulation of transcription by NF-kappaB, HPSE is also regulated posttranslationally by cathepsin L and that inhibition of heparanase activity directly affects HSV-2 release. We provide unique insights into the host mechanisms controlling HSV-2 egress and spread.
Anti-angiogenic drugs: direct anti-cancer agents with mitochondrial mechanisms of action.[Pubmed:29179466]
Oncotarget. 2017 Sep 13;8(51):88670-88688.
Components of the mitochondrial electron transport chain have recently gained much interest as potential therapeutic targets. Since mitochondria are essential for the supply of energy that is required for both angiogenic and tumourigenic activity, targeting the mitochondria represents a promising potential therapeutic approach for treating cancer. Here we investigate the established anti-angiogenesis drugs combretastatin A4, thalidomide, OGT 2115 and tranilast that we hypothesise are able to exert a direct anti-cancer effect in the absence of vasculature by targeting the mitochondria. Drug cytotoxicity was measured using the MTT assay. Mitochondrial function was measured in intact isolated mitochondria using polarography, fluorimetry and enzymatic assays to measure mitochondrial oxygen consumption, membrane potential and complex I-IV activities respectively. Combretastatin A4, OGT 2115 and tranilast were both shown to decrease mitochondrial oxygen consumption. OGT 2115 and tranilast decreased mitochondrial membrane potential and reduced complex I activity while combretastatin A4 and thalidomide did not. OGT 2115 inhibited mitochondrial complex II-III activity while combretastatin A4, thalidomide and tranilast did not. Combretastatin A4, thalidomide and OGT 2115 induced bi-phasic concentration-dependent increases and decreases in mitochondrial complex IV activity while tranilast had no evident effect. These data demonstrate that combretastatin A4, thalidomide, OGT 2115 and tranilast are all mitochondrial modulators. OGT 2115 and tranilast are both mitochondrial inhibitors capable of eliciting concentration-dependent reductions in cell viability by decreasing mitochondrial membrane potential and oxygen consumption.
Heparanase: a target for drug discovery in cancer and inflammation.[Pubmed:17339837]
Br J Pharmacol. 2007 May;151(1):1-14.
The remodelling of the extracellular matrix (ECM) has been shown to be highly upregulated in cancer and inflammation and is critically linked to the processes of invasion and metastasis. One of the key enzymes involved in specifically degrading the heparan sulphate (HS) component of the ECM is the endo-beta-glucuronidase enzyme heparanase. Processing of HS by heparanase releases both a host of bioactive growth factors anchored within the mesh of the ECM as well as defined fragments of HS capable of promoting cellular proliferation. The finding that heparanase is elevated in a wide variety of tumor types and is subsequently linked to the development of pathological processes has led to an explosion of therapeutic strategies to inhibit its enzyme activity. So far only one compound, the sulphated oligosaccharide PI88, which both inhibits heparanase activity and has effects on growth factor binding has reached clinical trials where it has shown to have promising efficacy. The scene has clearly been set however for a new generation of compounds, either specific to the enzyme or with dual roles, to emerge from the lab and enter the clinic. The aim of this review is to describe the current drug discovery status of small molecule, sugar and neutralising antibody inhibitors of heparanase enzyme activity. Potential strategies will also be discussed on the selection of suitable biomarker strategies for specific monitoring of in vivo heparanase inhibition which will be crucial for both animal model and clinical trial testing.


