SKF 89976A hydrochloridePotent GABA uptake inhibitor. Penetrates blood brain barrier CAS# 85375-15-1 |
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 85375-15-1 | SDF | Download SDF |
| PubChem ID | 6917797 | Appearance | Powder |
| Formula | C22H26ClNO2 | M.Wt | 371.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water with gentle warming and to 100 mM in DMSO | ||
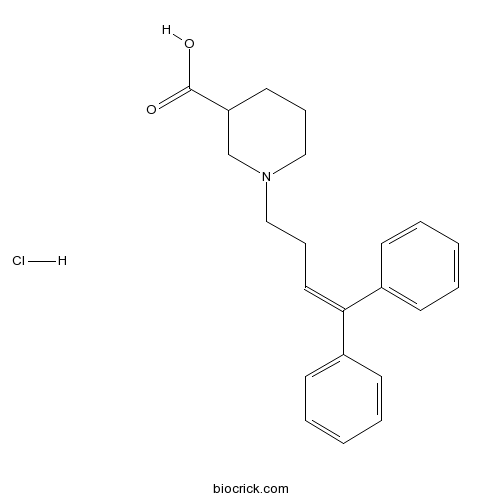
| Chemical Name | 1-(4,4-diphenylbut-3-enyl)piperidine-3-carboxylic acid;hydrochloride | ||
| SMILES | C1CC(CN(C1)CCC=C(C2=CC=CC=C2)C3=CC=CC=C3)C(=O)O.Cl | ||
| Standard InChIKey | SNGGBKYQZVAQKA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H25NO2.ClH/c24-22(25)20-13-7-15-23(17-20)16-8-14-21(18-9-3-1-4-10-18)19-11-5-2-6-12-19;/h1-6,9-12,14,20H,7-8,13,15-17H2,(H,24,25);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A potent GABA uptake inhibitor, selective for GAT-1 (IC50 values are 0.13, 550, 944 and 7210 μM for hGAT-1, rGAT-2, hGAT-3 and hBGT-1 respectively). Inhibits transport current competitively (Ki = 7 μM) and transmitter-gated current non-competitively (Ki = 0.03 nM). Able to pass the blood-brain barrier after systemic administration and is active in vivo. |

SKF 89976A hydrochloride Dilution Calculator

SKF 89976A hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6888 mL | 13.4441 mL | 26.8882 mL | 53.7765 mL | 67.2206 mL |
| 5 mM | 0.5378 mL | 2.6888 mL | 5.3776 mL | 10.7553 mL | 13.4441 mL |
| 10 mM | 0.2689 mL | 1.3444 mL | 2.6888 mL | 5.3776 mL | 6.7221 mL |
| 50 mM | 0.0538 mL | 0.2689 mL | 0.5378 mL | 1.0755 mL | 1.3444 mL |
| 100 mM | 0.0269 mL | 0.1344 mL | 0.2689 mL | 0.5378 mL | 0.6722 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,19-Epoxy-19R,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1328
CAS No.:85372-72-1
- 5,19-Epoxy-19S,25-dimethoxycucurbita-6,23-dien-3-ol
Catalog No.:BCN1329
CAS No.:85372-70-9
- SecinH3
Catalog No.:BCC7503
CAS No.:853625-60-2
- K03861
Catalog No.:BCC6537
CAS No.:853299-07-7
- Trichorabdal A
Catalog No.:BCN4404
CAS No.:85329-59-5
- Hythiemoside A
Catalog No.:BCN4403
CAS No.:853267-91-1
- Ajugalide D
Catalog No.:BCN3665
CAS No.:853247-65-1
- Ajugalide C
Catalog No.:BCN8015
CAS No.:853247-64-0
- PG 01
Catalog No.:BCC7820
CAS No.:853138-65-5
- Anthraquinone-1,5-disulfonic acid disodium salt
Catalog No.:BCC8833
CAS No.:853-35-0
- Dehydroepiandrosterone acetate
Catalog No.:BCC8929
CAS No.:853-23-6
- 20(21)-Dehydrolucidenic acid A
Catalog No.:BCN2940
CAS No.:852936-69-7
- (+)-AJ 76 hydrochloride
Catalog No.:BCC6747
CAS No.:85378-82-1
- NVP-BAG956
Catalog No.:BCC1813
CAS No.:853910-02-8
- OGT 2115
Catalog No.:BCC7458
CAS No.:853929-59-6
- Dasatinib hydrochloride
Catalog No.:BCC1517
CAS No.:854001-07-3
- Norcaesalpinin E
Catalog No.:BCN7006
CAS No.:854038-96-3
- (-)-Haplomyrfolin
Catalog No.:BCN3225
CAS No.:85404-48-4
- Ropivacaine mesylate
Catalog No.:BCC9137
CAS No.:854056-07-8
- Rilmenidine Phosphate
Catalog No.:BCC5637
CAS No.:85409-38-7
- S- (+)-Rolipram
Catalog No.:BCC2303
CAS No.:85416-73-5
- (R)-(-)-Rolipram
Catalog No.:BCC5429
CAS No.:85416-75-7
- Caffeic anhydride
Catalog No.:BCN3295
CAS No.:854237-32-4
- Ajugamarin chlorohydrin
Catalog No.:BCN3664
CAS No.:85447-27-4
Identification and selective inhibition of the channel mode of the neuronal GABA transporter 1.[Pubmed:16150932]
Mol Pharmacol. 2005 Dec;68(6):1728-35.
The function of GAT1, the transporter for the inhibitory neurotransmitter GABA, is characterized by expression in Xenopus laevis oocytes and measurements of GABA-induced uptake of [3H]GABA, 22Na+, and 36Cl-, and GABA-evoked currents under voltage-clamp conditions. N-[4,4-Diphenyl-3-butenyl]-nipecotic acid (SKF-89976-A), a specific inhibitor of GAT1, is used in our system as a pharmacological tool. The GABA-evoked current can be decomposed into a transport current, which is coupled to the GABA uptake, and a transmitter-gated current, which is uncoupled from the GABA uptake. The transport current results from a fixed stoichiometry of 1 GABA/2 Na+/1 Cl- transported during each cycle, as determined by radioactive tracer flux measurements. The transmitter-gated current is mediated by an Na+-conductance pathway. As a competitive inhibitor for GABA uptake, SKF-89976-A can separate the two current components. The GABA uptake is blocked with a K(I) value of approximately 7 microM, whereas the uncoupled transmitter-gated current is inhibited with a K(I) value of approximately 0.03 microM. Thus, the results of this study not only identify the transport mode and the channel mode of GAT1 but also raise the possibility of separating these components in a physiological environment.
Orally active and potent inhibitors of gamma-aminobutyric acid uptake.[Pubmed:2985785]
J Med Chem. 1985 May;28(5):653-60.
3-Pyrrolidineacetic acid (1a), certain piperidinecarboxylic acids--i.e., 3-piperidinecarboxylic acid (2a), 1,2,5,6-tetrahydro-3-pyridinecarboxylic acid (3a), and cis-4-hydroxy-3-piperidinecarboxylic acid (4a)--cis-3-aminocyclohexanecarboxylic acid (5a, cis-3-ACHC), and gamma-aminobutyric acid (6a, GABA) itself are among the most potent inhibitors of [3H]GABA uptake by neurons and glia in vitro. These hydrophilic amino acids, however, do not readily enter the central nervous system in pharmacologically significant amounts following peripheral administration. We now report that N-(4,4-diphenyl-3-butenyl)-3-piperidinecarboxylic acid (2b) is a specific GABA-uptake inhibitor that is more potent, more lipophilic and, in limited testing, as selective as 2a. Similar results were obtained with the N-(4,4-diphenyl-3-butenyl) derivatives of 1a, 3a, and 4a. By contrast, N-(4,4-diphenyl-3-butenyl) derivatives of 5a and 6a were not more potent than the parent amino acids and appear to inhibit GABA uptake, at least in part, by a nonselective mechanism of action. The N-(4,4-diphenyl-3-butenyl)amino acids 1b-4b exhibit anticonvulsant activity in rodents following oral or intraperitoneal administration [Yunger, L.M.; et al. J. Pharmacol. Exp. Ther. 1984, 228, 109].


