OrnidazoleCAS# 16773-42-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16773-42-5 | SDF | Download SDF |
| PubChem ID | 28061 | Appearance | Powder |
| Formula | C7H10ClN3O3 | M.Wt | 219.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ro 7-0207 | ||
| Solubility | DMSO : ≥ 100 mg/mL (455.31 mM) H2O : 10 mg/mL (45.53 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
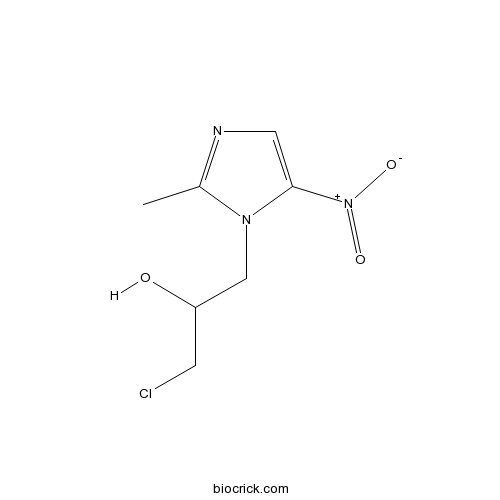
| Chemical Name | 1-chloro-3-(2-methyl-5-nitroimidazol-1-yl)propan-2-ol | ||
| SMILES | CC1=NC=C(N1CC(CCl)O)[N+](=O)[O-] | ||
| Standard InChIKey | IPWKIXLWTCNBKN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H10ClN3O3/c1-5-9-3-7(11(13)14)10(5)4-6(12)2-8/h3,6,12H,2,4H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ornidazole(Ro 7-0207) is a 5-nitroimidazole derivative with antiprotozoal and antibacterial properties against anaerobic bacteria.
Target: Antibacterial; Antiparasitic
Ornidazole is a drug that cures some protozoan infections. Ornidazole 1 g/day is effective for the prevention of recurrence of Crohn's disease after ileocolonic resection [1]. Ornidazole is converted to reduction products that interact with DNA to cause destruction of helical DNA structure and strand leading to a protein synthesis inhibition and cell death in susceptible organisms [2]. References: | |||||

Ornidazole Dilution Calculator

Ornidazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5531 mL | 22.7656 mL | 45.5311 mL | 91.0622 mL | 113.8278 mL |
| 5 mM | 0.9106 mL | 4.5531 mL | 9.1062 mL | 18.2124 mL | 22.7656 mL |
| 10 mM | 0.4553 mL | 2.2766 mL | 4.5531 mL | 9.1062 mL | 11.3828 mL |
| 50 mM | 0.0911 mL | 0.4553 mL | 0.9106 mL | 1.8212 mL | 2.2766 mL |
| 100 mM | 0.0455 mL | 0.2277 mL | 0.4553 mL | 0.9106 mL | 1.1383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ornidazole is a 5-nitroimidazole derivative with antiprotozoal and antibacterial properties against anaerobic bacteria.
- RS 39604 hydrochloride
Catalog No.:BCC5694
CAS No.:167710-87-4
- Boc-Asp(OtBu)-OH
Catalog No.:BCC3368
CAS No.:1676-90-0
- Z-Ser(tBu)-OH
Catalog No.:BCC2740
CAS No.:1676-75-1
- H-Glu(OBzl)-OH
Catalog No.:BCC2926
CAS No.:1676-73-9
- Benoxafos
Catalog No.:BCC5470
CAS No.:16759-59-4
- 11-Hydroxy-12-methoxyabietatriene
Catalog No.:BCN3253
CAS No.:16755-54-7
- PD-1/PD-L1 inhibitor 2
Catalog No.:BCC6520
CAS No.:1675203-84-5
- BADGE
Catalog No.:BCC7022
CAS No.:1675-54-3
- LY335979 (Zosuquidar 3HCL)
Catalog No.:BCC3878
CAS No.:167465-36-3
- Sophoracarpan B
Catalog No.:BCN6979
CAS No.:1674359-84-2
- Sophoracarpan A
Catalog No.:BCN6980
CAS No.:1674359-82-0
- H-Cys(Bzl)-OMe.HCl
Catalog No.:BCC2907
CAS No.:16741-80-3
- 3-Epidehydrotumulosic acid
Catalog No.:BCN3649
CAS No.:167775-54-4
- PD98059
Catalog No.:BCC1098
CAS No.:167869-21-8
- Rubelloside B
Catalog No.:BCN1099
CAS No.:167875-39-0
- Wilforol A
Catalog No.:BCN3064
CAS No.:167882-66-8
- ent-Kaurane-16beta,19,20-triol
Catalog No.:BCN7654
CAS No.:167898-32-0
- 2,5-Bis(4-diethylaminophenyl)-1,3,4-oxadiazole
Catalog No.:BCC8502
CAS No.:1679-98-7
- Crassanine
Catalog No.:BCN4073
CAS No.:16790-92-4
- 19(S)-Hydroxyconopharyngine
Catalog No.:BCN3976
CAS No.:16790-93-5
- Taxachitriene A
Catalog No.:BCN6952
CAS No.:167906-74-3
- Taxachitriene B
Catalog No.:BCN6951
CAS No.:167906-75-4
- Stigmasta-4,22-diene-3beta,6beta-diol
Catalog No.:BCN1533
CAS No.:167958-89-6
- Otophylloside B 4'''-O-beta-D-oleandropyranoside
Catalog No.:BCN7512
CAS No.:168001-54-5
The drug ornidazole inhibits photosynthesis in a different mechanism described for protozoa and anaerobic bacteria.[Pubmed:27647935]
Biochem J. 2016 Dec 1;473(23):4413-4426.
Ornidazole of the 5-nitroimidazole drug family is used to treat protozoan and anaerobic bacterial infections via a mechanism that involves preactivation by reduction of the nitro group, and production of toxic derivatives and radicals. Metronidazole, another drug family member, has been suggested to affect photosynthesis by draining electrons from the electron carrier ferredoxin, thus inhibiting NADP(+) reduction and stimulating radical and peroxide production. Here we show, however, that Ornidazole inhibits photosynthesis via a different mechanism. While having a minute effect on the photosynthetic electron transport and oxygen photoreduction, Ornidazole hinders the activity of two Calvin cycle enzymes, triose-phosphate isomerase (TPI) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Modeling of Ornidazole's interaction with ferredoxin of the protozoan Trichomonas suggests efficient electron tunneling from the iron-sulfur cluster to the nitro group of the drug. A similar docking site of Ornidazole at the plant-type ferredoxin does not exist, and the best simulated alternative does not support such efficient tunneling. Notably, TPI was inhibited by Ornidazole in the dark or when electron transport was blocked by dichloromethyl diphenylurea, indicating that this inhibition was unrelated to the electron transport machinery. Although TPI and GAPDH isoenzymes are involved in glycolysis and gluconeogenesis, Ornidazole's effect on respiration of photoautotrophs is moderate, thus raising its value as an efficient inhibitor of photosynthesis. The scarcity of Calvin cycle inhibitors capable of penetrating cell membranes emphasizes on the value of Ornidazole for studying the regulation of this cycle.
Ornidazole-induced fixed drug reaction on sole: case report and review of the literature.[Pubmed:27780370]
Cutan Ocul Toxicol. 2017 Sep;36(3):294-296.
BACKGROUND: Fixed drug eruption (FDE) is a special variant of drug reaction seen on skin or mucous membrane, and typically recurs at the same location. Ornidazole-induced FDE cases have been reported extremely rare. CASE: The 48-year-old female patient was diagnosed for Ornidazole-induced fixed drug reaction on the sole. The patient's history revealed that the lesion occurred for the third time in the last 6 months and she was administered Ornidazole tablet 3 times by the gynecologist for genitourinary tract infection. CONCLUSION: This report presents a case of fixed drug reaction located at the sole induced by Ornidazole use and a literature review.
Preparation, in Vitro and in Vivo Evaluations of Compound Calculus Bovis Sativus and Ornidazole Film.[Pubmed:27725435]
Biol Pharm Bull. 2016;39(10):1588-1595.
The aim of this study was to develop and to investigate a film of compound Calculus Bovis Sativus (CBS) and Ornidazole film. A uniform mucoadhesive film was herein successfully obtained by a film-forming solusion containing insoluable drug. This film, as a valid adjunct for the treatment of oral mucosal ulcer, consisted of two main drugs (CBS, Ornidazole) and three polymers (hydroxypropyl methyl cellulose, chitosan, poly(vinyl alcohol) (PVA)). The film was prepared with the film-forming suspension, using casting-solvent evaporation technique. The drug content, release behavior, swelling index and mucoadhesive properties of the film were detected. Then the effects of the prepared film on a glacial acetic acid-induced oral mucosal ulceration model of rabbits were evaluated. Moreover, the in vivo release of bilirubin and Ornidazole in saliva were also detected in the oral mucosae of healthy volunteers. The films showed favorable in vitro drug release behaviors and swelling properties. Mucosal wounds in the animals were significantly relieved. With the films well tolerated, the salivary concentrations of Ornidazole were maintained above the minimum inhibitory concentration against CBS for about 2 h. The compound CBS and Ornidazole film functioned better than the film only containing CBS and Ornidazole did. Therefore, it is a potentially efficient drug delivery system for the treatment of oral ulcers.
Preparation of monoclonal antibody and development of an indirect competitive enzyme-linked immunosorbent assay for ornidazole detection.[Pubmed:28372197]
Food Chem. 2017 Aug 15;229:439-444.
A monoclonal antibody (mAb) and an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) for Ornidazole (ONZ) detection were developed. ONZ was conjugated with cationic bovine serum albumin as a hapten to generate the artificial immunogens and coating antigens. BALB/c mice were immunized, and mAbs were obtained. The competitive inhibition curve of ic-ELISA was y=0.0438x(2)-0.2101x+0.2925, with R(2)=0.9941. The 50% inhibition concentration, the limit of detection, and limit of quality for ONZ were 0.15, 0.01, and 0.05microg/kg, respectively. The cross-reactivity of the mAbs to secnidazole was 0.33%. The recoveries were from 89.18% to 101.63% and the coefficient of variation was less than 7.15% in chicken, chicken liver, and honey samples, all of which had ONZ concentrations of 0.05 and 0.1mug/kg. Results showed that the ic-ELISA based on mAb could be used for the rapid detection for ONZ.


