OxypeucedaninCAS# 737-52-0 |
- (-)-Oxypeucedanin
Catalog No.:BCC9244
CAS No.:26091-73-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 737-52-0 | SDF | Download SDF |
| PubChem ID | 160544 | Appearance | White powder |
| Formula | C16H14O5 | M.Wt | 286.28 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in acetonitrile and chloroform | ||
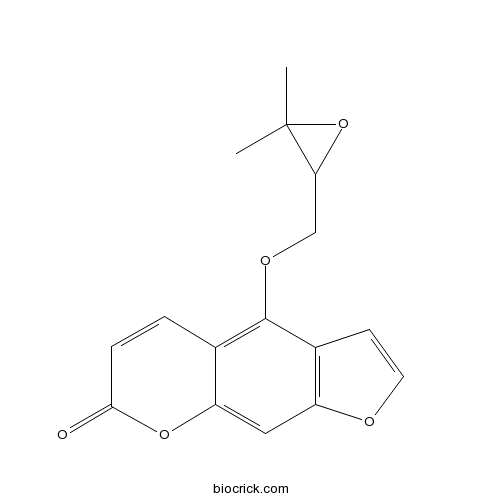
| Chemical Name | 4-[(3,3-dimethyloxiran-2-yl)methoxy]furo[3,2-g]chromen-7-one | ||
| SMILES | CC1(C(O1)COC2=C3C=CC(=O)OC3=CC4=C2C=CO4)C | ||
| Standard InChIKey | QTAGQHZOLRFCBU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O5/c1-16(2)13(21-16)8-19-15-9-3-4-14(17)20-12(9)7-11-10(15)5-6-18-11/h3-7,13H,8H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oxypeucedanin is a kind of open-channel blocker of the hKv1.5 channel and it prolongs the APD, it is an excellent candidate as an antiarrhythmic drug for atrial fibrillation.Oxypeucedanin has novel anticancer effect, mediated via induction of G2-M cell cycle arrest and apoptosis in human prostate carcinoma DU145 cells. |
| Targets | ERK | p38MAPK | PPAR | hKv1.5 channel | Caspase |
| In vitro | Effects of oxypeucedanin on global gene expression and MAPK signaling pathway in mouse neuroblastoma Neuro-2A cells.[Pubmed: 21425034]Planta Med. 2011 Sep;77(13):1512-8.Oxypeucedanin is a major coumarin aglycone that can be extracted from Ostericum koreanum. Coumarin aglycones have demonstrated various pharmacological effects, including anti-proliferation, anti-inflammation, and anti-pain. Effects of oxypeucedanin on hKv1.5 and action potential duration.[Pubmed: 15802805]Biol Pharm Bull. 2005 Apr;28(4):657-60.
|
| In vivo | Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: a comparative study.[Pubmed: 21273683]Pharmacol Rep. 2010 Nov-Dec;62(6):1231-6.The aim of this study was to determine and compare the anticonvulsant activities of four natural furanocoumarins [bergapten (5-methoxypsoralen), imperatorin (8-isopentenyloxypsoralen), Oxypeucedanin (5-epoxy-isopentenyloxypsoralen) and xanthotoxin (8-methoxypsoralen)] in the maximal electroshock-induced seizure test in mice. |
| Cell Research | Anti-tumor activity of oxypeucedanin from Ostericum koreanum against human prostate carcinoma DU145 cells.[Pubmed: 19322700]Acta Oncol. 2009;48(6):895-900.Oxypeucedanin has been reported to have various biological activities. We investigated the efficacy of a coumarin compound, Oxypeucedanin, from Ostericum koreanum against the human prostate carcinoma cell line DU145. |

Oxypeucedanin Dilution Calculator

Oxypeucedanin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4931 mL | 17.4654 mL | 34.9308 mL | 69.8617 mL | 87.3271 mL |
| 5 mM | 0.6986 mL | 3.4931 mL | 6.9862 mL | 13.9723 mL | 17.4654 mL |
| 10 mM | 0.3493 mL | 1.7465 mL | 3.4931 mL | 6.9862 mL | 8.7327 mL |
| 50 mM | 0.0699 mL | 0.3493 mL | 0.6986 mL | 1.3972 mL | 1.7465 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3493 mL | 0.6986 mL | 0.8733 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Onysilin
Catalog No.:BCN3379
CAS No.:73695-94-0
- Naringenin 5-rhamnoside
Catalog No.:BCN2747
CAS No.:
- HEPES
Catalog No.:BCC7590
CAS No.:7365-45-9
- H-Asp-OBzl
Catalog No.:BCC2882
CAS No.:7362-93-8
- p-Coumaric acid ethyl ester
Catalog No.:BCN4289
CAS No.:7362-39-2
- 5-Amino-1-(2-hydroxyethyl)pyrazole
Catalog No.:BCC8727
CAS No.:73616-27-0
- 1-(4-Methoxycinnamoyl)pyrrole
Catalog No.:BCN4027
CAS No.:736140-70-8
- Xylazine
Catalog No.:BCC5167
CAS No.:7361-61-7
- 3-Hydroxybenzylamine
Catalog No.:BCN1804
CAS No.:73604-31-6
- (-)-Bicuculline methobromide
Catalog No.:BCC6555
CAS No.:73604-30-5
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- 27-p-Coumaroyloxyursolic acid
Catalog No.:BCN4288
CAS No.:73584-67-5
- Acetylursolic acid
Catalog No.:BCN4290
CAS No.:7372-30-7
- Fmoc-Ser-OH
Catalog No.:BCC3541
CAS No.:73724-45-5
- Fmoc-Thr-OH
Catalog No.:BCC3549
CAS No.:73731-37-0
- Broussonin B
Catalog No.:BCN4593
CAS No.:73731-86-9
- Broussonin A
Catalog No.:BCN4592
CAS No.:73731-87-0
- Acanthoside B
Catalog No.:BCN4291
CAS No.:7374-79-0
- N-(4-Aminobenzoyl)-β-alanine
Catalog No.:BCC9056
CAS No.:7377-08-4
- 360A iodide
Catalog No.:BCC1308
CAS No.:737763-37-0
- Kihadanin B
Catalog No.:BCN3441
CAS No.:73793-68-7
- Trimethoprim
Catalog No.:BCC4873
CAS No.:738-70-5
- Bergamotine
Catalog No.:BCN2733
CAS No.:7380-40-7
- S-Sulfo-L-cysteine sodium salt
Catalog No.:BCC6558
CAS No.:7381-67-1
Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: a comparative study.[Pubmed:21273683]
Pharmacol Rep. 2010 Nov-Dec;62(6):1231-6.
The aim of this study was to determine and compare the anticonvulsant activities of four natural furanocoumarins [bergapten (5-methoxypsoralen), imperatorin (8-isopentenyloxypsoralen), Oxypeucedanin (5-epoxy-isopentenyloxypsoralen) and xanthotoxin (8-methoxypsoralen)] in the maximal electroshock-induced seizure test in mice. The anticonvulsant effects of bergapten, imperatorin, Oxypeucedanin, and xanthotoxin were evaluated at 15, 30, 60 and 120 min after their systemic (intraperitoneal) administration. Tonic hind limb extension (seizure activity) was evoked in adult albino Swiss mice by a current (sine-wave, 25 mA, 500 V, 50 Hz, 0.2 s stimulus duration) delivered via auricular electrodes. The time courses of protection by bergapten, imperatorin, Oxypeucedanin and xanthotoxin against maximal electroshock-induced seizures revealed that 300 mg/kg imperatorin and xanthotoxin (C-8 substituted derivatives of psoralen) exerted strong anticonvulsant activity, whereas 300 mg/kg bergapten and Oxypeucedanin (C-5 substituted derivatives of psoralen) did not produce any anticonvulsant activity in this model. In conclusion, imperatorin and xanthotoxin protected the animals against maximal electroshock-induced seizures, whereas bergapten and Oxypeucedanin, despite their chemical and structural similarities to xanthotoxin and imperatorin, exerted no anticonvulsant activity in this seizure test.
Effects of oxypeucedanin on hKv1.5 and action potential duration.[Pubmed:15802805]
Biol Pharm Bull. 2005 Apr;28(4):657-60.
A furocoumarin derivative, Oxypeucedanin, was purified from Angelica dahurica, and its effects on the human Kv1.5 (hKv1.5) channel and on the cardiac action potential duration (APD), were examined using the patch-clamp technique and the conventional microelectrode technique. Oxypeucedanin inhibited the hKv1.5 current in a concentration-dependent manner, with an IC(50) value of 76 nM, while it had no effect on human eag-related gene (HERG) current. Oxypeucedanin induced an initial fast decline of hKv1.5 current during depolarizations. The inhibition of hKv1.5 channel by Oxypeucedanin was voltage-dependent, especially at depolarizing pulses between -40 and 0 mV which corresponds to the voltage range of the channel's opening. Oxypeucedanin also slowed the deactivation time course, resulting in a tail crossover phenomenon. Additionally, Oxypeucedanin prolonged the APD of rat atrial and ventricular muscles in a dose-dependent manner. These results suggest that Oxypeucedanin is a kind of open-channel blocker of the hKv1.5 channel and it prolongs the APD; therefore, it is an excellent candidate as an antiarrhythmic drug for atrial fibrillation.
Anti-tumor activity of oxypeucedanin from Ostericum koreanum against human prostate carcinoma DU145 cells.[Pubmed:19322700]
Acta Oncol. 2009;48(6):895-900.
PURPOSE: Oxypeucedanin has been reported to have various biological activities. We investigated the efficacy of a coumarin compound, Oxypeucedanin, from Ostericum koreanum against the human prostate carcinoma cell line DU145. MATERIAL AND METHODS: Oxypeucedanin (C(16)H(14)O(5), mw: 286) was isolated through silica gel chromatography and characterized by NMR. The cells were treated with Oxypeucedanin (25, 50, and 100 microM) for 24-72 hours, and cell growth and death were then assayed. The cell cycle progression and apoptotic effects were also assessed by western blotting. RESULTS: Treatment with Oxypeucedanin inhibited cell growth and induced cell death in DU145 cells. Furthermore, Oxypeucedanin-induced cell growth inhibition was associated with an increase in G2-M arrest in cell cycle progression in DU145 cells in a dose and time-dependent manner. G2-M arrest by Oxypeucedanin was associated with decreased levels of cyclin A, cyclin B1, Cdc2, and pCdc2. Oxypeucedanin-induced cell death was associated with significant increases in apoptosis and cleaved caspase-3 and poly-(ADP-ribose) polymerase. CONCLUSION: These finding suggest a novel anticancer effect for Oxypeucedanin, mediated via induction of G2-M cell cycle arrest and apoptosis in human prostate carcinoma DU145 cells.
Effects of oxypeucedanin on global gene expression and MAPK signaling pathway in mouse neuroblastoma Neuro-2A cells.[Pubmed:21425034]
Planta Med. 2011 Sep;77(13):1512-8.
Oxypeucedanin is a major coumarin aglycone that can be extracted from Ostericum koreanum. Coumarin aglycones have demonstrated various pharmacological effects, including anti-proliferation, anti-inflammation, and anti-pain. In this study, in order to understand the pharmacological properties of Oxypeucedanin, we investigated global gene expression alteration in mouse neuroblastoma Neuro-2A cells. Results from the MTT assay indicated no decrease of cell viability up to 100 microM for 24 h. We measured gene expression profiles in Neuro-2A cells treated with either 10 microM or no Oxypeucedanin for 24 h. We selected 128 differentially expressed genes (DEGs) for comparison of gene expression profiles by Bonferroni-adjusted p values (p < 0.1). Analysis of Gene Ontology (GO) biological process terms using the DEGs demonstrated the importance of protein metabolism, particularly ribosomal protein synthesis and protein degradation, intramembrane protein trafficking, and electron transport. Treatment with Oxypeucedanin resulted in the downregulation of most DEGs for ribosomal protein synthesis and the electron transport chain (ETC). In contrast, most DEGs for protein degradation and cellular trafficking systems were upregulated. In addition, we found five upregulated DEGs for core and regulatory proteins involved in the mitogen-activated protein kinase (MAPK) signaling pathway. Independent translational validation of DEGs for MAPK signaling by immunoblot analysis showed consistent agreement with microarray data. Overall protein levels of Erk2 and p38MAPK were elevated, and their phosphorylated forms were also increased. These functional categories, based on transcriptional alteration and complicated modulation of MAPK signaling, might be underlying mechanisms responsible for the various pharmacological effects of Oxypeucedanin.


