PrasugrelADP receptor inhibitor CAS# 150322-43-3 |
- Clopidogrel
Catalog No.:BCC2497
CAS No.:120202-66-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 150322-43-3 | SDF | Download SDF |
| PubChem ID | 6918456 | Appearance | Powder |
| Formula | C20H20FNO3S | M.Wt | 373.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CS747;CS 747;CS-747;LY 640315;LY-640315;LY640315;Effient | ||
| Solubility | H2O : < 0.1 mg/mL (insoluble) | ||
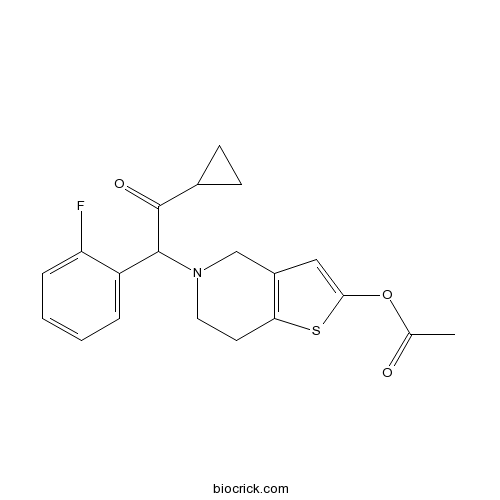
| Chemical Name | [5-[2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-6,7-dihydro-4H-thieno[3,2-c]pyridin-2-yl] acetate | ||
| SMILES | CC(=O)OC1=CC2=C(S1)CCN(C2)C(C3=CC=CC=C3F)C(=O)C4CC4 | ||
| Standard InChIKey | DTGLZDAWLRGWQN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20FNO3S/c1-12(23)25-18-10-14-11-22(9-8-17(14)26-18)19(20(24)13-6-7-13)15-4-2-3-5-16(15)21/h2-5,10,13,19H,6-9,11H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Irreversible P2Y12 antagonist; metabolised to active metabolite R-99224 (IC50 = 45 μM) irreversibly binds platelet P2Y12 receptors, inhibiting platelet activation. Antiplatelet and antithrombotic. Orally active and active in vivo. Note - inactive in vitro. |

Prasugrel Dilution Calculator

Prasugrel Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6778 mL | 13.389 mL | 26.7781 mL | 53.5561 mL | 66.9452 mL |
| 5 mM | 0.5356 mL | 2.6778 mL | 5.3556 mL | 10.7112 mL | 13.389 mL |
| 10 mM | 0.2678 mL | 1.3389 mL | 2.6778 mL | 5.3556 mL | 6.6945 mL |
| 50 mM | 0.0536 mL | 0.2678 mL | 0.5356 mL | 1.0711 mL | 1.3389 mL |
| 100 mM | 0.0268 mL | 0.1339 mL | 0.2678 mL | 0.5356 mL | 0.6695 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Prasugrel is an inhibitor of platelet aggregation that is used to decrease the risk of myocardial infarction and stroke in patients with acute coronary syndromes. Prasugrel has been linked to mild and transient serum enzyme elevations during therapy and to rare instances of hypersensitivity reactions accompanied by mild liver injury
- Z-Aib-OH
Catalog No.:BCC3150
CAS No.:15030-72-5
- H-Aib-Ome.HCl
Catalog No.:BCC2671
CAS No.:15028-41-8
- Ledipasvir D-tartrate
Catalog No.:BCC4047
CAS No.:1502654-87-6
- Fmoc-p-amino-benzoic acid,Fmoc-4-Abz-OH
Catalog No.:BCC2622
CAS No.:15026-42-1
- Fmoc-2-Abz-OH
Catalog No.:BCC3204
CAS No.:150256-42-1
- 12-Deoxo-12alpha-acetoxyelliptone
Catalog No.:BCN4803
CAS No.:150226-21-4
- Trigothysoid N
Catalog No.:BCN6881
CAS No.:1501943-08-3
- BIM 23056
Catalog No.:BCC5824
CAS No.:150155-61-6
- Crucigasterin 225
Catalog No.:BCN1786
CAS No.:150151-85-2
- Crucigasterin 275
Catalog No.:BCN1776
CAS No.:150151-84-1
- Crucigasterin 277
Catalog No.:BCN1777
CAS No.:150151-83-0
- 11,13-Dihydroivalin
Catalog No.:BCN4705
CAS No.:150150-61-1
- Cyclopropyl 2-fluorobenzyl ketone
Catalog No.:BCC8923
CAS No.:150322-73-9
- Quipazine dimaleate
Catalog No.:BCC6727
CAS No.:150323-78-7
- threo-7-O-Methylguaiacylglycerol beta-coniferyl ether
Catalog No.:BCN6929
CAS No.:150333-85-0
- Sivelestat sodium salt
Catalog No.:BCC2368
CAS No.:150374-95-1
- SR 49059
Catalog No.:BCC7324
CAS No.:150375-75-0
- MRS 2690
Catalog No.:BCC7514
CAS No.:15039-58-4
- Pemetrexed disodium
Catalog No.:BCN2179
CAS No.:150399-23-8
- o-Methoxycinnamaldehyde
Catalog No.:BCN3860
CAS No.:1504-74-1
- L-NIL hydrochloride
Catalog No.:BCC5706
CAS No.:150403-89-7
- epi-Eudesmol
Catalog No.:BCN1670
CAS No.:15051-81-7
- Fmoc-Lys(Dde)-OH
Catalog No.:BCC3518
CAS No.:150629-67-7
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
The in vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties.[Pubmed:10742300]
Br J Pharmacol. 2000 Apr;129(7):1439-46.
1. CS-747 is a novel antiplatelet agent that generates an active metabolite, R-99224, in vivo. CS-747 itself was totally inactive in vitro. This study examined in vivo pharmacological profiles of CS-747 after single oral administration to rats. 2. Orally administered CS-747 (0.3 - 10 mg kg(-1)) partially but significantly decreased [(3)H]-2-methylthio-ADP binding to rat platelets. CS-747 (3 mg kg(-1), p.o.) treatment neutralized ADP-induced decreases of cyclic AMP concentrations induced by prostaglandin E(1), suggesting that metabolites of CS-747 interfere with G(i)-linked P2T receptor. 3. CS-747 (0.3 and 3 mg kg(-1), p.o.) markedly inhibited ex vivo washed platelet aggregation in response to ADP but not to thrombin. CS-747 also exhibited a marked inhibition of ADP-induced ex vivo platelet aggregation in PRP with a rapid onset (<0.5 h) and long duration (>3 days) of action (ED(50) at 4 h=1.2 mg kg(-1)). 4. R-99224 (IC(50)=45 microM) inhibited in vitro PRP aggregation in a concentration-related manner. 5. CS-747 prevented thrombus formation in a dose-related manner with an ED(50) value of 0.68 mg kg(-1). CS-747 was more potent than clopidogrel (6.2 mg kg(-1)) and ticlopidine (>300 mg kg(-1)). 6. CS-747, clopidogrel, and ticlopidine prolonged the bleeding time. The order of potency of these agents in this activity was the same as that in antiaggregatory and antithrombotic activities. 7. These findings indicate that CS-747 is an orally active and a potent antiplatelet and antithrombotic agent with a rapid onset and long duration of action, and warrants clinical evaluations of the agent.
Clopidogrel, prasugrel, or ticagrelor use and clinical outcome in patients with acute coronary syndrome: A nationwide long-term registry analysis from 2009 to 2014.[Pubmed:28262344]
Int J Cardiol. 2017 May 15;235:61-66.
BACKGROUND: The beneficial use of dual antiplatelet therapy (DAPT) with acetylsalicylic acid (ASA) and P2Y12 oinhibitors has been established for patients after acute coronary syndrome (ACS). However, the optimal duration of DAPT is under debate. The aim of the present study was to investigate the long-term utilization and clinical outcome of clopidogrel, Prasugrel, and ticagrelor in patients with ACS from 2009 to 2014 in Austria. METHODS: We analysed data from 13 Austrian health insurance funds for the years 2009 to 2014, on 72,676 patients with a hospital discharge diagnosis of ACS. The primary end point was recurrence of ACS or death >30days after the index event. RESULTS: 32,830 subjects received a prescription of a P2Y12 inhibitor within 30days after the index ACS. 18,640 (56.8%) subjects were discharged with clopidogrel, 6683 (20.4%) with Prasugrel, and 7507 (22.9%) with ticagrelor, respectively. Data from 32,174 patients with 4975 events during a median follow-up period of 24.9months were available for survival analysis. The cumulative incidence for recurrence of ACS or death at two years was 18.7% in patients receiving clopidogrel, and 8.7% and 12.0% in those receiving Prasugrel or ticagrelor, respectively. CONCLUSION: Utilization of P2Y12 inhibitors in patients with ACS was consistent with guideline recommendations. Prasugrel and ticagrelor are increasingly used in ACS patients and associated with a lower number of recurrence of ACS or death compared to clopidogrel. However, clopidogrel was predominantly used in older patients with more co-morbidities.
Prasugrel suppresses development of lithium-induced nephrogenic diabetes insipidus in mice.[Pubmed:28233082]
Purinergic Signal. 2017 Jun;13(2):239-248.
Previously, we localized ADP-activated P2Y12 receptor (R) in rodent kidney and showed that its blockade by clopidogrel bisulfate (CLPD) attenuates lithium (Li)-induced nephrogenic diabetes insipidus (NDI). Here, we evaluated the effect of Prasugrel (PRSG) administration on Li-induced NDI in mice. Both CLPD and PRSG belong to the thienopyridine class of ADP receptor antagonists. Groups of age-matched adult male B6D2 mice (N = 5/group) were fed either regular rodent chow (CNT), or with added LiCl (40 mmol/kg chow) or PRSG in drinking water (10 mg/kg bw/day) or a combination of LiCl and PRSG for 14 days and then euthanized. Water intake and urine output were determined and blood and kidney tissues were collected and analyzed. PRSG administration completely suppressed Li-induced polydipsia and polyuria and significantly prevented Li-induced decreases in AQP2 protein abundance in renal cortex and medulla. However, PRSG either alone or in combination with Li did not have a significant effect on the protein abundances of NKCC2 or NCC in the cortex and/or medulla. Immunofluorescence microscopy revealed that PRSG administration prevented Li-induced alterations in cellular disposition of AQP2 protein in medullary collecting ducts. Serum Li, Na, and osmolality were not affected by the administration of PRSG. Similar to CLPD, PRSG administration had no effect on Li-induced increase in urinary Na excretion. However, unlike CLPD, PRSG did not augment Li-induced increase in urinary arginine vasopressin (AVP) excretion. Taken together, these data suggest that the pharmacological inhibition of P2Y12-R by the thienopyridine group of drugs may potentially offer therapeutic benefits in Li-induced NDI.
Prasugrel for Japanese patients with acute coronary syndrome in short-term clinical practice (PRASFIT-Practice I): a postmarketing observational study.[Pubmed:28213685]
Cardiovasc Interv Ther. 2018 Apr;33(2):135-145.
Data on Prasugrel use in Japanese patients are limited to phase II/III clinical trials. This early postmarketing observational study evaluated the safety and efficacy of short-term Prasugrel use in patients with acute coronary syndrome (ACS) in real-world clinical settings in Japan. From May 2014 to January 2015, we enrolled consecutive patients with ACS requiring percutaneous coronary intervention in each institution. Each patient started Prasugrel treatment >/=1 month before the end of the study period. Safety outcomes included incidence rates of adverse drug reactions (ADRs) and bleeding adverse events (AEs). Efficacy outcomes were incidence rates of cardiovascular events (including major adverse cardiovascular events [MACE]). Case report forms were collected from 749 patients, 732 of whom were eligible for the safety and efficacy analysis sets. Approximately 95% of patients had a Prasugrel loading/maintenance dose of 20 mg/3.75 mg/day. The incidences of ADRs and bleeding AEs were 8.6 and 6.4%, respectively. Twelve patients experienced major bleeding AEs; approximately 60% (seven patients) of which were gastrointestinal disorders. The incidence of bleeding AEs was significantly higher primarily in patients of female sex, aged >/=75 years, with low body weight (Prasugrel treatment, and 3.1% at the end of the study period. This short-term study indicated that Prasugrel treatment at loading/maintenance doses of 20 mg/3.75 mg/day was safe and effective in Japanese ACS patients in an acute setting. CLINICAL TRIAL REGISTRATION: This study is registered at http://www.umin.ac.jp/ctr/ under the identifier UMIN000014699.


