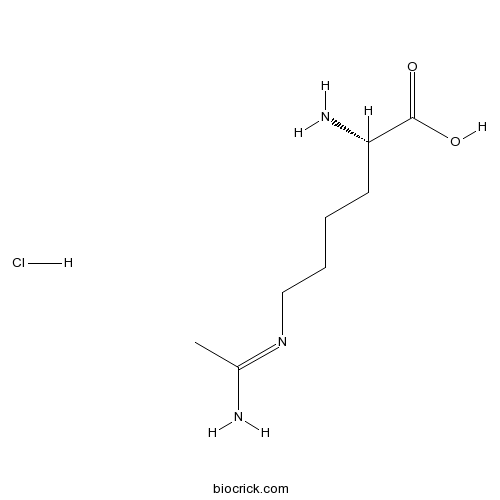
L-NIL hydrochlorideSelective iNOS inhibitor CAS# 150403-89-7 |
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 150403-89-7 | SDF | Download SDF |
| PubChem ID | 9794509 | Appearance | Powder |
| Formula | C8H18ClN3O2 | M.Wt | 223.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (2S)-2-amino-6-(1-aminoethylideneamino)hexanoic acid;hydrochloride | ||
| SMILES | CC(=NCCCCC(C(=O)O)N)N.Cl | ||
| Standard InChIKey | HJYWSATZDBEAOS-FJXQXJEOSA-N | ||
| Standard InChI | InChI=1S/C8H17N3O2.ClH/c1-6(9)11-5-3-2-4-7(10)8(12)13;/h7H,2-5,10H2,1H3,(H2,9,11)(H,12,13);1H/t7-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective inhibitor of inducible nitric oxide synthase (IC50 = 3.3 μM). |

L-NIL hydrochloride Dilution Calculator

L-NIL hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4703 mL | 22.3514 mL | 44.7027 mL | 89.4055 mL | 111.7568 mL |
| 5 mM | 0.8941 mL | 4.4703 mL | 8.9405 mL | 17.8811 mL | 22.3514 mL |
| 10 mM | 0.447 mL | 2.2351 mL | 4.4703 mL | 8.9405 mL | 11.1757 mL |
| 50 mM | 0.0894 mL | 0.447 mL | 0.8941 mL | 1.7881 mL | 2.2351 mL |
| 100 mM | 0.0447 mL | 0.2235 mL | 0.447 mL | 0.8941 mL | 1.1176 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- o-Methoxycinnamaldehyde
Catalog No.:BCN3860
CAS No.:1504-74-1
- Pemetrexed disodium
Catalog No.:BCN2179
CAS No.:150399-23-8
- MRS 2690
Catalog No.:BCC7514
CAS No.:15039-58-4
- SR 49059
Catalog No.:BCC7324
CAS No.:150375-75-0
- Sivelestat sodium salt
Catalog No.:BCC2368
CAS No.:150374-95-1
- threo-7-O-Methylguaiacylglycerol beta-coniferyl ether
Catalog No.:BCN6929
CAS No.:150333-85-0
- Quipazine dimaleate
Catalog No.:BCC6727
CAS No.:150323-78-7
- Cyclopropyl 2-fluorobenzyl ketone
Catalog No.:BCC8923
CAS No.:150322-73-9
- Prasugrel
Catalog No.:BCC1089
CAS No.:150322-43-3
- Z-Aib-OH
Catalog No.:BCC3150
CAS No.:15030-72-5
- H-Aib-Ome.HCl
Catalog No.:BCC2671
CAS No.:15028-41-8
- Ledipasvir D-tartrate
Catalog No.:BCC4047
CAS No.:1502654-87-6
- epi-Eudesmol
Catalog No.:BCN1670
CAS No.:15051-81-7
- Fmoc-Lys(Dde)-OH
Catalog No.:BCC3518
CAS No.:150629-67-7
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- 5-Methoxycanthin-6-one
Catalog No.:BCN3326
CAS No.:15071-56-4
- Calyxamine B
Catalog No.:BCN1671
CAS No.:150710-72-8
- Drynachromoside A
Catalog No.:BCN7891
CAS No.:1507388-29-5
- Gap19
Catalog No.:BCC5599
CAS No.:1507930-57-5
- Oxybutynin chloride
Catalog No.:BCC4149
CAS No.:1508-65-2
- Tropicamide
Catalog No.:BCC4574
CAS No.:1508-75-4
- Retigabine
Catalog No.:BCC6427
CAS No.:150812-12-7
- Retigabine dihydrochloride
Catalog No.:BCC1890
CAS No.:150812-13-8
- 2,3,24-Trihydroxyolean-12-en-28-oic acid
Catalog No.:BCN1559
CAS No.:150821-16-2
Primary murine microglia are resistant to nitric oxide inhibition of indoleamine 2,3-dioxygenase.[Pubmed:20451602]
Brain Behav Immun. 2010 Nov;24(8):1249-53.
Indoleamine 2,3-dioxygenase (IDO) is an intracellular heme-containing enzyme that is activated by proinflammatory cytokines, including interferon-gamma (IFNgamma), and metabolizes tryptophan along the kynurenine pathway. Activation of murine macrophages induces not only IDO but also nitric oxide synthase (iNOS), and the ensuing production of nitric oxide (NO) inhibits IDO. To determine the sensitivity of primary cultures of murine microglia to NO, microglia were stimulated with recombinant murine IFNgamma (1 ng/ml) and lipopolysaccharide (LPS) (10 ng/ml). This combination of IFNgamma+LPS synergized to produce maximal amounts of nitrite as early as 16h. Steady-state mRNAs for both iNOS and IDO were significantly increased by IFNgamma+LPS at 4h post-treatment, followed by an increase in IDO enzymatic activity at 24h. Murine microglia (>95% CD11b(+)) were pretreated with the iNOS inhibitor, L-NIL hydrochloride, at a dose (30 muM) that completely abrogated production of nitrite. L-NIL had no effect on IDO mRNA at 4h or IDO enzymatic activity at 24h following stimulation with IFNgamma+LPS. These data establish that IDO regulation in murine microglia is not restrained by NO, thereby permitting the accumulation of kynurenine and its downstream metabolites in the central nervous system.
Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase.[Pubmed:7537678]
Eur J Pharmacol. 1995 Jan 24;273(1-2):15-24.
Adjuvant-induced arthritis is a model of chronic inflammation that exhibits several pathological changes similar to those occurring in rheumatoid arthritis, an autoimmune disease in humans characterized by chronic inflammation of the joints. We have examined the role of inducible nitric oxide synthase in producing the pathological changes associated with adjuvant-induced arthritis. Plasma nitrite concentrations were maximally elevated 14 days following adjuvant administration compared to untreated control animals. Arthritic changes in the paw were first observed between days 10-12 and were maximally elevated 21 days following adjuvant administration. Inducible nitric oxide synthase immunoreactivity was found localized in the synovial tissue from adjuvant-treated rats, while untreated controls exhibited no inducible nitric oxide synthase staining. Two selective inducible nitric oxide synthase inhibitors, aminoguanidine and N-iminoethyl-L-lysine, suppressed the increase in plasma nitrite levels and joint inflammation associated with adjuvant-induced arthritis in a dose-dependent manner. N-Iminoethyl-L-lysine attenuated the inducible nitric oxide synthase immunoreactivity in adjuvant-treated rats. Blood pressure was not affected by the highest dose of N-iminoethyl-L-lysine administered in the drinking water, indicating a lack of inhibition of constitutive nitric oxide synthase.
L-N6-(1-iminoethyl)lysine: a selective inhibitor of inducible nitric oxide synthase.[Pubmed:7525961]
J Med Chem. 1994 Nov 11;37(23):3886-8.
L-N6-(1-Iminoethyl)lysine (L-NIL) has been synthesized and is shown to be both a potent and selective inhibitor of mouse inducible nitric oxide synthase (miNOS). L-NIL has an IC50 of 3.3 microM for miNOS compared to an IC50 of 92 microM for rat brain constitutive NOS indicating that L-NIL is 28-fold more selective for inducible NOS. L-N5-(1-Iminoethyl)ornithine (L-NIO), which differs from L-NIL by having one less methylene group, has very similar potency for inducible NOS, but lacks selectivity. DL-N7-(1-Iminoethyl)homolysine was also synthesized and found to be substantially less potent than L-NIL or L-NIO, with intermediate selectivity for inducible NOS. These data suggest that L-NIL may be useful as a selective inhibitor of inducible NOS for determining the role of this enzyme in disease models.


