Fmoc-Lys(Dde)-OHCAS# 150629-67-7 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

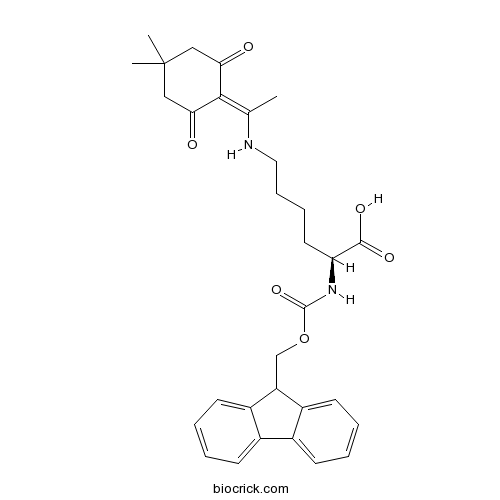
| Cas No. | 150629-67-7 | SDF | Download SDF |
| PubChem ID | 10918490 | Appearance | Powder |
| Formula | C31H36N2O6 | M.Wt | 532.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-6-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethylamino]-2-(9H-fluoren-9-ylmethoxycarbonylamino)hexanoic acid | ||
| SMILES | CC(=C1C(=O)CC(CC1=O)(C)C)NCCCCC(C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24 | ||
| Standard InChIKey | ZPSRBXWVBNVFTO-VWLOTQADSA-N | ||
| Standard InChI | InChI=1S/C31H36N2O6/c1-19(28-26(34)16-31(2,3)17-27(28)35)32-15-9-8-14-25(29(36)37)33-30(38)39-18-24-22-12-6-4-10-20(22)21-11-5-7-13-23(21)24/h4-7,10-13,24-25,32H,8-9,14-18H2,1-3H3,(H,33,38)(H,36,37)/t25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Lys(Dde)-OH Dilution Calculator

Fmoc-Lys(Dde)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8776 mL | 9.3879 mL | 18.7758 mL | 37.5516 mL | 46.9395 mL |
| 5 mM | 0.3755 mL | 1.8776 mL | 3.7552 mL | 7.5103 mL | 9.3879 mL |
| 10 mM | 0.1878 mL | 0.9388 mL | 1.8776 mL | 3.7552 mL | 4.694 mL |
| 50 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.751 mL | 0.9388 mL |
| 100 mM | 0.0188 mL | 0.0939 mL | 0.1878 mL | 0.3755 mL | 0.4694 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Lys(Dde)-OH
- epi-Eudesmol
Catalog No.:BCN1670
CAS No.:15051-81-7
- L-NIL hydrochloride
Catalog No.:BCC5706
CAS No.:150403-89-7
- o-Methoxycinnamaldehyde
Catalog No.:BCN3860
CAS No.:1504-74-1
- Pemetrexed disodium
Catalog No.:BCN2179
CAS No.:150399-23-8
- MRS 2690
Catalog No.:BCC7514
CAS No.:15039-58-4
- SR 49059
Catalog No.:BCC7324
CAS No.:150375-75-0
- Sivelestat sodium salt
Catalog No.:BCC2368
CAS No.:150374-95-1
- threo-7-O-Methylguaiacylglycerol beta-coniferyl ether
Catalog No.:BCN6929
CAS No.:150333-85-0
- Quipazine dimaleate
Catalog No.:BCC6727
CAS No.:150323-78-7
- Cyclopropyl 2-fluorobenzyl ketone
Catalog No.:BCC8923
CAS No.:150322-73-9
- Prasugrel
Catalog No.:BCC1089
CAS No.:150322-43-3
- Z-Aib-OH
Catalog No.:BCC3150
CAS No.:15030-72-5
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- 5-Methoxycanthin-6-one
Catalog No.:BCN3326
CAS No.:15071-56-4
- Calyxamine B
Catalog No.:BCN1671
CAS No.:150710-72-8
- Drynachromoside A
Catalog No.:BCN7891
CAS No.:1507388-29-5
- Gap19
Catalog No.:BCC5599
CAS No.:1507930-57-5
- Oxybutynin chloride
Catalog No.:BCC4149
CAS No.:1508-65-2
- Tropicamide
Catalog No.:BCC4574
CAS No.:1508-75-4
- Retigabine
Catalog No.:BCC6427
CAS No.:150812-12-7
- Retigabine dihydrochloride
Catalog No.:BCC1890
CAS No.:150812-13-8
- 2,3,24-Trihydroxyolean-12-en-28-oic acid
Catalog No.:BCN1559
CAS No.:150821-16-2
- Nitenpyram
Catalog No.:BCC5559
CAS No.:150824-47-8
- EGF816
Catalog No.:BCC6428
CAS No.:1508250-71-2
Pitfalls in the synthesis of fluorescent methotrexate oligopeptide conjugates.[Pubmed:27357306]
Amino Acids. 2016 Nov;48(11):2599-2604.
Methotrexate (MTX) conjugates with poly[Lys(DL-Alam)] based polymeric polypeptides are efficient against Leishmania donovani parasite infection, but the mechanism of the effect is not known yet. We prepared therefore the 5(6)-carboxyfluorescein (Cf) labeled oligopeptide [Cf-K(AaAa)] (a: D-alanine, A: L-alanine) and the corresponding MTX conjugates [Cf-K(MTX-AaAa)] as model compounds for structure-activity experiments. The conjugate aimed to be synthesized with solid phase methodology on MBHA resin with Boc strategy, using Fmoc-Lys(Boc)-OH. However, various side reactions were identified. Here we report three problems observed during the synthesis as well as solutions developed by us: (1) unexpected cyclopeptide-formation with the lactone-carboxylic group of the Cf was detected, when Cf was attached to the alpha-amino group of the Lys residue on solid phase. This was avoided by changing the order of Cf incorporation with using Fmoc/Dde strategy. Alternatively, we have built the peptide with Fmoc strategy on solid phase first and performed the labeling with Cf-OSu subsequently in solution. (2) During HF cleavage of the protected conjugates, MTX was demonstrated to form adducts with anisole and p-cresol scavengers, and the TMSOTf cleavage methodology was also found to be inadequate due to the large number of side products formed. We report here that using Fmoc/Dde strategy is an appropriate method to circumvent the cleavage with HF or TMSOTf. (3) During the coupling of MTX with oligopeptide, structural and stereo isomers are formed. We have described here the suitable conditions of HPLC separation of these products.
Strategies for the synthesis of labeled peptides.[Pubmed:19499054]
J Biomol Tech. 2000 Dec;11(4):155-65.
Labeled peptides synthesized by core facilities are frequently used by researchers for following trafficking of a peptide, for binding studies, to determine substrate specificity, and for receptor cross-linking studies.The membership of the Association of Biomolecular Resource Facilities was asked to participate in a study focusing on synthesis of a biotin-labeled peptide, and it was suggested that a new strategy, using Rink amide 4-methylbenzhydrylamine resin coupled with Fmoc-Lys(Dde)-OH, be used.This strategy can be used for addition of a variety of labels other than biotin and should prove useful to core facilities. Comparison of the new strategy to other strategies was performed. Biotin labeling has long been assumed to be routine and specific. Despite the assumed routine nature of synthesizing biotinylated peptides, 9 of the 34 samples submitted did not contain any of the correct product. Although synthesis using Fmoc-Lys(Dde)-OH plus biotin generally gave the highest yields, other approaches also yielded a high percentage of the correct product.Therefore, the various strategies are generally comparable. The major advantage of this new approach is that other labels such as fluorescein, dansyl groups, methyl coumarin, and potentially fluorophores and quenchers used for fluorescence resonance energy transfer (FRET) can be directly incorporated into peptides.


