SR 49059Selective, orally active vasopressin V1A receptor antagonist CAS# 150375-75-0 |
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 150375-75-0 | SDF | Download SDF |
| PubChem ID | 60943 | Appearance | Powder |
| Formula | C28H27Cl2N3O7S | M.Wt | 620.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Relcovaptan | ||
| Solubility | Soluble to 30 mM in DMSO | ||
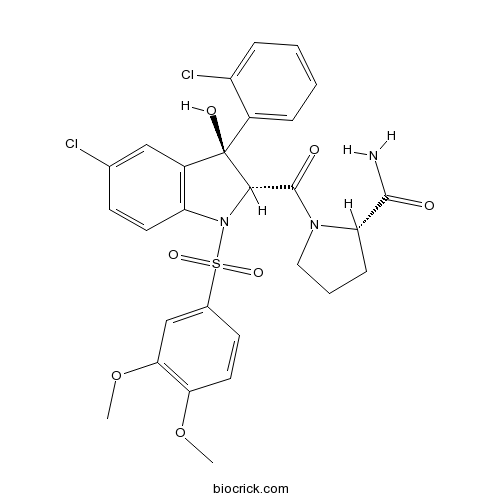
| Chemical Name | (2S)-1-[(2R,3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4-dimethoxyphenyl)sulfonyl-3-hydroxy-2H-indole-2-carbonyl]pyrrolidine-2-carboxamide | ||
| SMILES | COC1=C(C=C(C=C1)S(=O)(=O)N2C(C(C3=C2C=CC(=C3)Cl)(C4=CC=CC=C4Cl)O)C(=O)N5CCCC5C(=O)N)OC | ||
| Standard InChIKey | CEBYCSRFKCEUSW-NAYZPBBASA-N | ||
| Standard InChI | InChI=1S/C28H27Cl2N3O7S/c1-39-23-12-10-17(15-24(23)40-2)41(37,38)33-21-11-9-16(29)14-19(21)28(36,18-6-3-4-7-20(18)30)25(33)27(35)32-13-5-8-22(32)26(31)34/h3-4,6-7,9-12,14-15,22,25,36H,5,8,13H2,1-2H3,(H2,31,34)/t22-,25-,28+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective non-peptide vasopressin V1A receptor antagonist; devoid of agonist activity. Displays high affinity and efficacy at both rat (Ki = 1.6 nM) and human (Ki = 1.1 - 6.3 nM) V1A receptors. Potently antagonizes arginine vasopressin-induced effects in vitro (IC50 = 3.7 nM for inhibition of human platelet aggregation) and is orally active in vivo. |

SR 49059 Dilution Calculator

SR 49059 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6116 mL | 8.058 mL | 16.116 mL | 32.2321 mL | 40.2901 mL |
| 5 mM | 0.3223 mL | 1.6116 mL | 3.2232 mL | 6.4464 mL | 8.058 mL |
| 10 mM | 0.1612 mL | 0.8058 mL | 1.6116 mL | 3.2232 mL | 4.029 mL |
| 50 mM | 0.0322 mL | 0.1612 mL | 0.3223 mL | 0.6446 mL | 0.8058 mL |
| 100 mM | 0.0161 mL | 0.0806 mL | 0.1612 mL | 0.3223 mL | 0.4029 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sivelestat sodium salt
Catalog No.:BCC2368
CAS No.:150374-95-1
- threo-7-O-Methylguaiacylglycerol beta-coniferyl ether
Catalog No.:BCN6929
CAS No.:150333-85-0
- Quipazine dimaleate
Catalog No.:BCC6727
CAS No.:150323-78-7
- Cyclopropyl 2-fluorobenzyl ketone
Catalog No.:BCC8923
CAS No.:150322-73-9
- Prasugrel
Catalog No.:BCC1089
CAS No.:150322-43-3
- Z-Aib-OH
Catalog No.:BCC3150
CAS No.:15030-72-5
- H-Aib-Ome.HCl
Catalog No.:BCC2671
CAS No.:15028-41-8
- Ledipasvir D-tartrate
Catalog No.:BCC4047
CAS No.:1502654-87-6
- Fmoc-p-amino-benzoic acid,Fmoc-4-Abz-OH
Catalog No.:BCC2622
CAS No.:15026-42-1
- Fmoc-2-Abz-OH
Catalog No.:BCC3204
CAS No.:150256-42-1
- 12-Deoxo-12alpha-acetoxyelliptone
Catalog No.:BCN4803
CAS No.:150226-21-4
- Trigothysoid N
Catalog No.:BCN6881
CAS No.:1501943-08-3
- MRS 2690
Catalog No.:BCC7514
CAS No.:15039-58-4
- Pemetrexed disodium
Catalog No.:BCN2179
CAS No.:150399-23-8
- o-Methoxycinnamaldehyde
Catalog No.:BCN3860
CAS No.:1504-74-1
- L-NIL hydrochloride
Catalog No.:BCC5706
CAS No.:150403-89-7
- epi-Eudesmol
Catalog No.:BCN1670
CAS No.:15051-81-7
- Fmoc-Lys(Dde)-OH
Catalog No.:BCC3518
CAS No.:150629-67-7
- Tolvaptan
Catalog No.:BCC5096
CAS No.:150683-30-0
- 5-Methoxycanthin-6-one
Catalog No.:BCN3326
CAS No.:15071-56-4
- Calyxamine B
Catalog No.:BCN1671
CAS No.:150710-72-8
- Drynachromoside A
Catalog No.:BCN7891
CAS No.:1507388-29-5
- Gap19
Catalog No.:BCC5599
CAS No.:1507930-57-5
- Oxybutynin chloride
Catalog No.:BCC4149
CAS No.:1508-65-2
Effects of nonpeptide V(1) vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model.[Pubmed:12468809]
Stroke. 2002 Dec;33(12):3033-7.
BACKGROUND AND PURPOSE: Cerebral edema develops very early after the onset of focal cerebral ischemia and may be a major factor in early disability after an acute ischemic stroke. There have been very limited studies on the usefulness of antiedemic agents as neuroprotective agents in the setting of focal cerebral ischemia. We tested the neuroprotective effects of a new potent nonpeptide vasopressin receptor V(1) antagonist, SR-49059, in a focal embolic stroke model in rats. METHODS: Focal ischemic injury was induced by embolizing a preformed clot into the middle cerebral artery (MCA). Infarction volume was measured at 48 hours after the MCA occlusion. Neurological deficits, ischemic brain edema, seizure activity, and mortality and hemorrhage rates were also documented. RESULTS: Treatment with SR-49059 (2 mg/kg), initiated immediately after MCA occlusion, significantly reduced infarction volume (P<0.05) measured at 48 hours after the arterial occlusion. In animals in which the treatment was delayed for 1 hour after MCA occlusion, infarction volume was also reduced significantly (P<0.05). Infarction volume in the rats that received the drug at 3 or 6 hours after MCA occlusion was not different from that in the vehicle-treated group. Treatment with SR-49059, when started early after the arterial occlusion, also reduced neurological deficits and ischemic brain edema. Injection of drug at a higher dose (30 mg/kg) also reduced infarction volume and improved functional recovery but was not superior to the lower dose (2 mg/kg) when the drug was administrated at 1 hour after MCA occlusion. CONCLUSIONS: Our data show that the selective vasopressin receptor antagonist SR-49059 is a potent neuroprotective agent when used early after onset of arterial occlusion in an embolic focal ischemia model in rats. Further studies are needed in stroke models to better understand its neuroprotective properties when used alone or in combination with thrombolysis.
A nonpeptide vasopressin V(1a) receptor antagonist, SR 49059, does not prevent cisplatin-induced emesis in piglets.[Pubmed:11468030]
Fundam Clin Pharmacol. 2001 Jun;15(3):189-200.
We determined the pharmacological and the antiemetic properties of SR 49059, a selective nonpeptide V(1a) receptor antagonist, on cisplatin-induced emesis in the piglet. Firstly, we clearly demonstrate that SR 49059 is a potent V(1a) receptor antagonist in vitro and in vivo in the piglet. In binding studies, [3H]-SR 49059 exhibited high affinity for V(1a) receptors in piglet liver membranes (K(d) of 0.76 +/- 0.12 nM and B(max) of 138 +/- 22 fmol/mg prot.). In vivo, in decerebrate piglets, SR 49059 (1 mg/kg iv) antagonized AVP (500 ng/kg iv)-induced hypertension for at least 150 min and also blocked, for at least 270 min at 3 mg/kg iv, the pressor responses to exogenous LVP. After single and repeated iv or icv administration, we studied the antiemetic properties of SR 49059 on cisplatin-induced emesis in piglets. Animals receiving an emetic dose of cisplatin (5.5 mg/kg, iv) were observed continuously for 60 h. Piglets acting as controls were iv administered with vehicle 15 min prior to cisplatin infusion (T0(-15min)), while experimental animals received a single iv administration of SR 49059 at the dose of 1 or 3 mg/kg. In additional piglets, we administered SR 49059 (3 mg/kg) every 12 h from T0(-15min) to T48(-15min) (cumulative dose, 15 mg/kg). Another set of animals - observed only during the acute phase - was administered with SR 49059 (10 mg/kg) every 3 h from T0(-15min) to T15(-15min) (cumulative dose, 60 mg/kg). Lastly, 10 piglets were given a bilateral icv injection of SR 49059 (500 microg and 1500 microg/side) 1 h prior to cisplatin infusion. In all groups treated with SR 49059, the latency of the first emetic episode and the incidence of vomiting during the acute, the delayed and the cumulative phases remained statistically similar to that observed in controls, suggesting that V(1a) receptors are not involved in the onset and completion of nausea and vomiting.
The effect of relcovaptan (SR 49059), an orally active vasopressin V1a receptor antagonist, on uterine contractions in preterm labor.[Pubmed:15823830]
Gynecol Endocrinol. 2005 Feb;20(2):104-9.
Relcovaptan (SR 49059) is a non-peptide, orally active vasopressin V1a receptor inhibitor. The effect on uterine contractions in 18 women with preterm labor in pregnancy weeks 32-36 was assessed in a double-blind investigation. The inclusion criterion was at least four regular uterine contractions over 30 min as measured by external tocodynamometry. Twelve patients received at random a single oral dose of 400 mg relcovaptan and six received placebo, and contractions were monitored up to 6 h thereafter. Rescue medication (beta-adrenoceptor-stimulating drug) was allowed after 2 h. Before drug administration a mean (+/- SE) of 8.2 +/- 1.4 and 9.7 +/- 1.6 contractions/30 min were recorded in the relcovaptan- and placebo-treated groups, respectively. In the former group, the frequency of uterine contractions started to decrease within the first half hour, and 1.5-2 h after dosing it was steady at 3.2 +/- 0.9 contractions/30 min. Correspondingly, after placebo, 7.8 +/- 2.2 contractions/30 min were recorded, a statistically significant difference (p = 0.017). The activity in the relcovaptan-treated women remained low, whereas in the placebo group inhibited uterine contractions were observed only in women receiving 'rescue' tocolytic treatment. It is concluded that relcovaptan inhibits preterm labor.
Inhibitory effects of SR 49059 on oxytocin-and vasopressin-induced uterine contractions in non-pregnant women.[Pubmed:14678081]
Acta Obstet Gynecol Scand. 2004 Jan;83(1):12-8.
BACKGROUND: Compounds that block uterine oxytocin and vasopressin V1a receptors have a therapeutic potential in preterm labor and primary dysmenorrhoea. The orally active vasopressin V1a receptor antagonist, SR49059, inhibits the effect of vasopressin on human uterine activity in vivo, but the influence on the response to oxytocin is unknown. METHODS: In a placebo-controlled, double-blind, parallel-group, four-dose comparison, the inhibitory effect of SR 49059 on oxytocin- and vasopressin-induced uterine contractions in humans was investigated. Sixteen healthy female subjects, who had previously undergone sterilization with tubal ligation, participated in intrauterine pressure recordings at one of the first 3 days of bleeding of two menstrual cycles. Intravenous bolus injections of 10 pmol/kg body weight of vasopressin (Period 1) and of 50 pmol/kg body weight of oxytocin (Period 2) were given 1 h before and 1, 2 and 4 h after oral administration of 0 (placebo), 25, 75 or 200 mg of SR 49059. The area between the recording curve and zero level of intrauterine pressure (AUC) was calculated. Vital signs as well as urine and plasma safety parameters were measured. The plasma concentrations of oxytocin, vasopressin and the study drug were also estimated. RESULTS: The plasma concentrations of SR 49059 appeared to be dose related, with mean maximal values of 62.0, 163.7 and 468.0 ng/ml in the 25, 75 and 200 mg dose groups, respectively, in Period 1 with vasopressin and 34.4, 116.7 and 418.0 ng/mL, respectively, in Period 2 with oxytocin. Tmax was observed at about 1 h. The cumulative AUC over 50 min after vasopressin injection per se was significantly higher than that after oxytocin in spite of a five times lower dose and lower plasma concentrations. Pretreatment by SR 49059 caused a dose-related reduction in AUCs for vasopressin, whereas no such effect was seen for oxytocin. With vasopressin as an agonist, a lower diastolic blood pressure was observed in all SR 49059 treatment groups, but not with oxytocin. CONCLUSIONS: The much higher potency of vasopressin compared with oxytocin on uterine activity in non-pregnant women at menstruation was confirmed. SR 49059 dose-dependently inhibits vasopressin-induced contractions, whereas such an effect was not seen with the present doses of SR 49059 and oxytocin. A marked reduction by SR 49059 of diastolic blood pressure after vasopressin injection was observed, indicating an inhibition by this compound of vascular vasopressin receptors.
Identification of the binding sites of the SR49059 nonpeptide antagonist into the V1a vasopressin receptor using sulfydryl-reactive ligands and cysteine mutants as chemical sensors.[Pubmed:12869559]
J Biol Chem. 2003 Oct 10;278(41):40010-9.
To identify the binding site of the human V1a vasopressin receptor for the selective nonpeptide antagonist SR49059, we have developed a site-directed irreversible labeling strategy that combines mutagenesis of the receptor and use of sulfydryl-reactive ligands. Based on a three-dimensional model of the antagonist docked into the receptor, hypothetical ligand-receptor interactions were investigated by replacing the residues potentially involved in the binding of the antagonist into cysteines and designing analogues of SR49059 derivatized with isothiocyanate or alpha-chloroacetamide moieties. The F225C, F308C, and K128C mutants of the V1a receptor were expressed in COS-7 or Chinese hamster ovary cells, and their pharmacological properties toward SR49059 and its sulfydryl-reactive analogues were analyzed. We demonstrated that treatment of the F225C mutant with the isothiocyanate-derivative compound led to dose-dependent inhibition of the residual binding of the radio-labeled antagonist [125I]HO-LVA. This inhibition is probably the consequence of a covalent irreversible chemical modification, which is only possible when close contacts and optimal orientations exist between reactive groups created both on the ligand and the receptor. This result validated the three-dimensional model hypothesis. Thus, we propose that residue Phe225, located in transmembrane domain V, directly participates in the binding of the V1a-selective nonpeptide antagonist SR49059. This conclusion is in complete agreement with all our previous data on the definition of the agonist/antagonist binding to members of the oxytocin/vasopressin receptor family.
Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors.[Pubmed:8392086]
J Clin Invest. 1993 Jul;92(1):224-31.
SR 49059, a new potent and selective orally active, nonpeptide vasopressin (AVP) antagonist has been characterized in several in vitro and in vivo models. SR 49059 showed high affinity for V1a receptors from rat liver (Ki = 1.6 +/- 0.2) and human platelets, adrenals, and myometrium (Ki ranging from 1.1 to 6.3 nM). The previously described nonpeptide V1 antagonist, OPC-21268, was almost inactive in human tissues at concentrations up to 100 microM. SR 49059 exhibited much lower affinity (two orders of magnitude or more) for AVP V2 (bovine and human), V1b (human), and oxytocin (rat and human) receptors and had no measurable affinity for a great number of other receptors. In vitro, AVP-induced contraction of rat caudal artery was competitively antagonized by SR 49059 (pA2 = 9.42). Furthermore, SR 49059 inhibited AVP-induced human platelet aggregation with an IC50 value of 3.7 +/- 0.4 nM, while OPC-21268 was inactive up to 20 microM. In vivo, SR 49059 inhibited the pressor response to exogenous AVP in pithed rats (intravenous) and in conscious normotensive rats (intravenous and per os) with a long duration of action (> 8 h at 10 mg/kg p.o). In all the biological assays used, SR 49059 was devoid of any intrinsic agonistic activity. Thus, SR 49059 is the most potent and selective nonpeptide AVP V1a antagonist described so far, with marked affinity, selectivity, and efficacy toward both animal and human receptors. With this original profile, SR 49059 constitutes a powerful tool for exploring the therapeutical usefulness of a selective V1a antagonist.


