PseudoakuammigineCAS# 2447-70-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2447-70-3 | SDF | Download SDF |
| PubChem ID | 5478100 | Appearance | Powder |
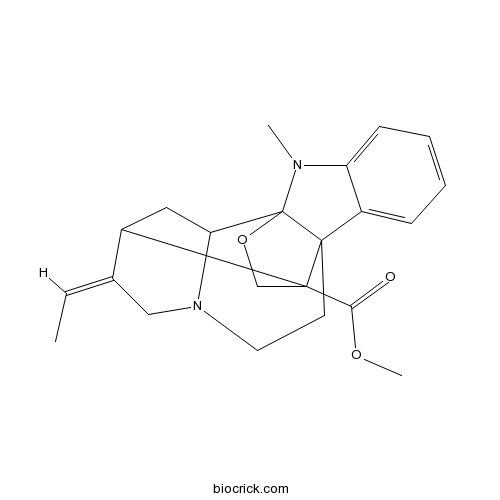
| Formula | C22H26N2O3 | M.Wt | 366.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC=C1CN2CCC34C5=CC=CC=C5N(C36C2CC1C4(CO6)C(=O)OC)C | ||
| Standard InChIKey | HAGBWVNSVWLTKY-LNKIKWGQSA-N | ||
| Standard InChI | InChI=1S/C22H26N2O3/c1-4-14-12-24-10-9-21-15-7-5-6-8-17(15)23(2)22(21)18(24)11-16(14)20(21,13-27-22)19(25)26-3/h4-8,16,18H,9-13H2,1-3H3/b14-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Pseudoakuammigine exhibits opioid analgesic activity in vivo. 2. Pseudoakuammigine exhibits anti-inflammatory and analgesic actions, the analgesic actions are mediated via interaction with opioid receptors. |
| Targets | Immunology & Inflammation related |

Pseudoakuammigine Dilution Calculator

Pseudoakuammigine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7285 mL | 13.6426 mL | 27.2851 mL | 54.5703 mL | 68.2128 mL |
| 5 mM | 0.5457 mL | 2.7285 mL | 5.457 mL | 10.9141 mL | 13.6426 mL |
| 10 mM | 0.2729 mL | 1.3643 mL | 2.7285 mL | 5.457 mL | 6.8213 mL |
| 50 mM | 0.0546 mL | 0.2729 mL | 0.5457 mL | 1.0914 mL | 1.3643 mL |
| 100 mM | 0.0273 mL | 0.1364 mL | 0.2729 mL | 0.5457 mL | 0.6821 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sulfadoxine
Catalog No.:BCC4726
CAS No.:2447-57-6
- Sanguinarine
Catalog No.:BCN5102
CAS No.:2447-54-3
- 4-Chlorodehydromethyltestosterone
Catalog No.:BCC8704
CAS No.:2446-23-3
- Daphnetin dimethyl ether
Catalog No.:BCN2735
CAS No.:2445-80-9
- Nonivamide
Catalog No.:BCN2325
CAS No.:2444-46-4
- 3,5,7,15-Tetraacetoxy-9-nicotinoyloxy-6(17),11-jatrophadien-14-one
Catalog No.:BCN6592
CAS No.:244277-75-6
- LFM-A13
Catalog No.:BCC6472
CAS No.:244240-24-2
- JTC-801
Catalog No.:BCC3800
CAS No.:244218-51-7
- Celaphanol A
Catalog No.:BCN5101
CAS No.:244204-40-8
- Pulchinenoside E2
Catalog No.:BCN8186
CAS No.:244202-36-6
- L-748,337
Catalog No.:BCC7475
CAS No.:244192-94-7
- Taxumairol R
Catalog No.:BCN6939
CAS No.:244167-04-2
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Z-D-Phe-OH
Catalog No.:BCC2755
CAS No.:2448-45-5
- Bryonolic acid
Catalog No.:BCN5103
CAS No.:24480-45-3
- Isochlorogenic acid A
Catalog No.:BCN5908
CAS No.:2450-53-5
- Acetylshikonin
Catalog No.:BCN2665
CAS No.:24502-78-1
- Dimethylacrylshikonin
Catalog No.:BCN2310
CAS No.:23444-70-4
- 19-Oxocinobufagin
Catalog No.:BCN8229
CAS No.:24512-59-2
- 19-Oxocinobufotalin
Catalog No.:BCN8233
CAS No.:24512-60-5
- Gardenoside
Catalog No.:BCN2383
CAS No.:24512-62-7
- Geniposide
Catalog No.:BCN5104
CAS No.:24512-63-8
- Monomethyl kolavate
Catalog No.:BCN5105
CAS No.:24513-41-5
- 3,21-Dihydroxy-14-serraten-16-one
Catalog No.:BCN5106
CAS No.:24513-51-7
Opioid activity of alkaloids extracted from Picralima nitida (fam. Apocynaceae).[Pubmed:9683021]
Eur J Pharmacol. 1998 May 29;350(1):101-8.
Extracts of the seeds of Picralima nitida (fam. Apocynaceae) have been reported to have opioid analgesic activity. In this investigation, isolated tissue bioassays and radioligand binding assays have been used to determine the opioid activity of five alkaloids--akuammidine, akuammine, akuammicine, akuammigine and Pseudoakuammigine--extracted from the seeds of P. nitida. Akuammidine showed a preference for mu-opioid binding sites with Ki values of 0.6, 2.4 and 8.6 microM at mu-, delta- and kappa-opioid binding sites, respectively. The agonist actions of akuammidine in the mouse-isolated vas deferens were antagonised by naloxone and the mu-opioid receptor selective antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) confirming an action at mu-opioid receptors. In contrast, akuammine also showed highest affinity for mu-opioid binding sites (Ki 0.5 microM) but was an antagonist at mu-opioid receptors with a pK(B) of 5.7 against the selective mu-opioid receptor agonist [D-Ala2,MePhe4,Gly-ol5]enkephalin (DAMGO). Akuammicine has the highest affinity for kappa-opioid binding sites (Ki 0.2 microM) and was a full agonist at kappa-opioid receptors in the guinea pig ileum preparation but a partial kappa-opioid receptor agonist in the vasa deferentia of the mouse and the rabbit. Akuammigine and Pseudoakuammigine showed little or no efficacy in the opioid bioassays. None of the alkaloids had significant activity for opioid receptor-like binding sites (ORL1-binding sites) with Ki values >> 10 microM. These data show that some alkaloids extracted from the medicinal plant P. nitida possess varying degrees of agonist and antagonist activity at opioid receptors but possess neither high affinity nor selectivity for mu-, delta- or kappa-opioid receptors or the ORL1-receptor.
Current progress in the chemistry and pharmacology of akuammiline alkaloids.[Pubmed:12871110]
Curr Med Chem. 2003 Sep;10(18):1891-915.
Akuammiline alkaloids are a family of monoterpene indole alkaloids of renewed medicinal interest. These bases act as ligands for a heterogeneous group of molecular targets and, consequently, display a wide variety of pharmacological activities. For example, Pseudoakuammigine (2) exhibits opioid activity in vivo, echitamine (4) has been reported to have promising cytotoxic activity, and corymine (121) behaves as an antagonist of the glycine receptor. Oddly enough, these alkaloids have not raised enough interest in the organic synthesis community, remaining inaccessible; even the entry to their pentacyclic framework continues elusive. Recently, several akuammiline bases have been isolated and identified including bisindole alkaloids, such as vingramine (103) or rausutrine (110), which incorporate akuammiline-type subunits. This review covers the advances in the chemistry and pharmacology of akuammiline alkaloids reported within the last ten years.
Pseudo-akuammigine, an alkaloid from Picralima nitida seeds, has anti-inflammatory and analgesic actions in rats.[Pubmed:12020930]
J Ethnopharmacol. 2002 Jun;81(1):73-9.
Pseudo-akuammigine, an alkaloid from Picralima nitida seed extract was investigated for anti-inflammatory and analgesic actions using the carrageenan-induced rat paw oedema and the rat tail flick. The alkaloid, at 1.0, 5.0 and 50 mgkg(-1)(,) dose-dependently inhibited the mean maximal paw swelling attained during 6 h to 78.2+/-2.1, 74.7+/-4.3 and 59.5+/-2.3% of the mean control value respectively when administered p.o. 1 h before induction of oedema. At the same dose levels, the total paw swelling over the 6-h period was also significantly (P<0.05) reduced to 83.2+/-9.7, 73.0+/-5.0 and 55.8+/-8.3% of the mean control response respectively. When administered after induction of oedema, psi-akuammigine (5.0 mgkg(-1)) significantly (P<0.05) reduced established rat paw swelling to 82.8+/-4.6% of the control response after 5 h. As an analgesic, psi-akuammigine was 3.5 and 1.6 times less potent than morphine and indomethacin respectively. The ED(50) values were Morphine (2.9 microM), psi-akuammigine (10 microM) and indomethacin (6.3 microM). Naloxone (1.0 mgkg(-1)) significantly (P<0.05) antagonised the analgesic action of the alkaloid by 35.8+/-6.8%. Pseudo-akuammigine therefore exhibits anti-inflammatory and analgesic actions. The analgesic actions are mediated via interaction with opioid receptors.


