JTC-801KOR-3 (NOP) receptor antagonist CAS# 244218-51-7 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

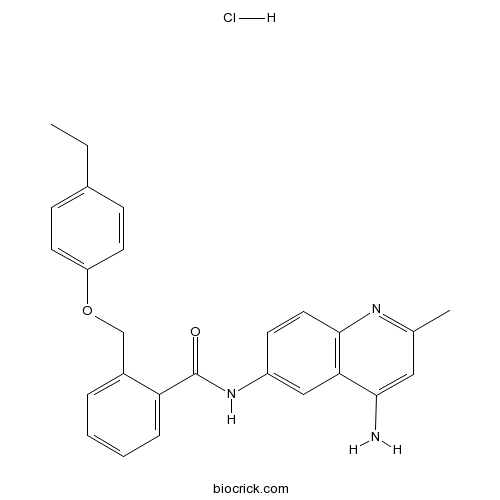
| Cas No. | 244218-51-7 | SDF | Download SDF |
| PubChem ID | 5311339 | Appearance | Powder |
| Formula | C26H26ClN3O2 | M.Wt | 447.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 0.33 mg/mL (0.74 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-(4-amino-2-methylquinolin-6-yl)-2-[(4-ethylphenoxy)methyl]benzamide;hydrochloride | ||
| SMILES | CCC1=CC=C(C=C1)OCC2=CC=CC=C2C(=O)NC3=CC4=C(C=C3)N=C(C=C4N)C.Cl | ||
| Standard InChIKey | NQLIYKXNAXKMBL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H25N3O2.ClH/c1-3-18-8-11-21(12-9-18)31-16-19-6-4-5-7-22(19)26(30)29-20-10-13-25-23(15-20)24(27)14-17(2)28-25;/h4-15H,3,16H2,1-2H3,(H2,27,28)(H,29,30);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity, selective NOP receptor antagonist (Ki = 8.2 nM). Displays approximately 12.5-, 129- and 1055-fold selectivity over human μ-, κ- and δ-opioid receptors respectively. Exhibits anti-nociceptive effects in acute pain models in vivo. Orally active. |

JTC-801 Dilution Calculator

JTC-801 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2323 mL | 11.1617 mL | 22.3234 mL | 44.6468 mL | 55.8086 mL |
| 5 mM | 0.4465 mL | 2.2323 mL | 4.4647 mL | 8.9294 mL | 11.1617 mL |
| 10 mM | 0.2232 mL | 1.1162 mL | 2.2323 mL | 4.4647 mL | 5.5809 mL |
| 50 mM | 0.0446 mL | 0.2232 mL | 0.4465 mL | 0.8929 mL | 1.1162 mL |
| 100 mM | 0.0223 mL | 0.1116 mL | 0.2232 mL | 0.4465 mL | 0.5581 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
JTC 801 is a high affinity, selective KOR-3 (NOP) receptor antagonist (Ki = 8.2 nM). Displays approximately 12.5-, 129- and 1055-fold selectivity over human μ-, κ- and δ-opioid receptors respectively.
- Celaphanol A
Catalog No.:BCN5101
CAS No.:244204-40-8
- Pulchinenoside E2
Catalog No.:BCN8186
CAS No.:244202-36-6
- L-748,337
Catalog No.:BCC7475
CAS No.:244192-94-7
- Taxumairol R
Catalog No.:BCN6939
CAS No.:244167-04-2
- L-798,106
Catalog No.:BCC7654
CAS No.:244101-02-8
- Beta-Rotunol
Catalog No.:BCN6628
CAS No.:24405-57-0
- (+)-Epipinoresinol
Catalog No.:BCN3255
CAS No.:24404-50-0
- beta-D-glucose
Catalog No.:BCN8171
CAS No.:492-61-5
- TAK-242
Catalog No.:BCC1977
CAS No.:243984-11-4
- TAK-242 S enantiomer
Catalog No.:BCC1978
CAS No.:243984-10-3
- TCS 401
Catalog No.:BCC2469
CAS No.:243967-42-2
- Cnicin
Catalog No.:BCN8546
CAS No.:24394-09-0
- LFM-A13
Catalog No.:BCC6472
CAS No.:244240-24-2
- 3,5,7,15-Tetraacetoxy-9-nicotinoyloxy-6(17),11-jatrophadien-14-one
Catalog No.:BCN6592
CAS No.:244277-75-6
- Nonivamide
Catalog No.:BCN2325
CAS No.:2444-46-4
- Daphnetin dimethyl ether
Catalog No.:BCN2735
CAS No.:2445-80-9
- 4-Chlorodehydromethyltestosterone
Catalog No.:BCC8704
CAS No.:2446-23-3
- Sanguinarine
Catalog No.:BCN5102
CAS No.:2447-54-3
- Sulfadoxine
Catalog No.:BCC4726
CAS No.:2447-57-6
- Pseudoakuammigine
Catalog No.:BCN4812
CAS No.:2447-70-3
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Z-D-Phe-OH
Catalog No.:BCC2755
CAS No.:2448-45-5
- Bryonolic acid
Catalog No.:BCN5103
CAS No.:24480-45-3
- Isochlorogenic acid A
Catalog No.:BCN5908
CAS No.:2450-53-5
Anti-allodynic and anti-hyperalgesic effects of nociceptin receptor antagonist, JTC-801, in rats after spinal nerve injury and inflammation.[Pubmed:15763246]
Eur J Pharmacol. 2005 Mar 14;510(3):223-8.
The effects of nociceptin/orphanin FQ (N/OFQ) peptide receptor antagonist JTC-801 on allodynia and hyperalgesia were examined in rats in order to explore the involvement of N/OFQ system in these pathological pain states. Tactile allodynia induced by L5/L6 spinal nerve ligation was reversed by both systemic (3-30 mg/kg) and spinal (22.5 and 45 pg) JTC-801 in a dose-dependent manner. Concerning hyperalgesia induced by formalin injection into the hindpaw, JTC-801 dose-dependently suppressed the second phase, but not the first phase, of the licking behavior. Furthermore, systemic JTC-801 reduced Fos-like immunoreactivity in the dorsal horn of the spinal cord (laminae I/II). In conclusion, N/OFQ receptor antagonist JTC-801 exerted anti-allodynic and anti-hyperalgesic effects in rats, suggesting that N/OFQ system might be involved in the modulation of neuropathic pain and inflammatory hyperalgesia.
Nociceptin receptor antagonist JTC-801 inhibits nitrous oxide-induced analgesia in mice.[Pubmed:19444578]
J Anesth. 2009;23(2):301-3.
The mechanism of the analgesic effect of nitrous oxide (N(2)O) has not been completely clarified. Although we have reported that the analgesic effect of N(2)O was significantly decreased in nociceptin-orphanin FQ (N/OFQ) receptor (NOP)-deficient mice, the effect of nociceptin receptor antagonists on N(2)O-induced analgesia has not been reported. In this investigation, we examined the effect of the NOP antagonist JTC-801 on N(2)O-induced analgesia in 129Sv mice by the writhing test and tail flick test, and demonstrated that the analgesic effect of N(2)O was suppressed by the intraperitoneal administration of JTC-801.
NOP receptor antagonist, JTC-801, blocks cannabinoid-evoked hypothermia in rats.[Pubmed:17512052]
Neuropeptides. 2007 Aug;41(4):239-47.
The present study used the endpoint of hypothermia to investigate cannabinoid and nociceptin/orphanin FQ (N/OFQ) interactions in conscious animals. Prior work has established that cannabinoids produce hypothermia by activating central cannabinoid CB(1) receptors. The administration of N/OFQ into the brain also causes significant hypothermia. Those data suggest a link between cannabinoid CB(1) receptors and N/OFQ peptide (NOP) receptors in the production of hypothermia. Therefore, we determined if NOP receptor activation is required for cannabinoid-evoked hypothermia and if cannabinoid CB(1) receptor activation is necessary for N/OFQ-induced hypothermia. In actual experiments, a cannabinoid agonist, WIN 55212-2 (2.5, 5, and 10 mg/kg, i.p.), caused significant hypothermia in male Sprague-Dawley rats (200-225 g). A NOP receptor antagonist, JTC-801 (1 mg/kg, i.p.), did not affect body temperature. For combined administration, JTC-801 (1 mg/kg, i.p.) blocked a significant proportion of the hypothermia caused by each dose of WIN 55212-2 (2.5, 5, and 10 mg/kg, i.p.). JTC-801 (1 mg/kg, i.p.) also blocked the hypothermia caused by another cannabinoid agonist, CP-55, 940 (1 mg/kg, i.p.). In separate experiments, the direct administration of N/OFQ (9 microg/rat, i.c.v.) into the brain produced significant hypothermia. The hypothermic effect of N/OFQ was blocked by JTC-801 (1 mg/kg, i.p.) but not by a selective cannabinoid CB(1) antagonist, SR 141716A (5 mg/kg, i.m.). The finding that a NOP receptor antagonist abolishes a significant percentage of cannabinoid-induced hypothermia suggests that NOP receptor activation is required for cannabinoids to produce hypothermia. This interaction, quantitated in the present study, is the first evidence that NOP receptors mediate a cannabinoid-induced effect in conscious animals.
Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder.[Pubmed:24666365]
Br J Pharmacol. 2015 Jan;172(2):571-82.
BACKGROUND AND PURPOSE: Single-prolonged stress (SPS), a rat model of post-traumatic stress disorder (PTSD), also induces long-lasting hyperalgesia associated with hypocortisolism and elevated nociceptin/orphanin FQ (N/OFQ) levels in serum and CSF. Here, we determined the effect of JTC-801 (N-(4-amino-2-methylquinolin-6-yl)-2-(4-ethylphenoxymethyl) benzamide monohydrochloride), a nociceptin/orphanin FQ peptide (NOP) receptor antagonist, on symptoms of pain and anxiety in rats after SPS exposure, and examined N/OFQ-NOP receptor system changes. EXPERIMENTAL APPROACH: Male Sprague Dawley rats received JTC-801 (6 mg kg(-1) i.p., once daily) during days 7-21 of SPS. The ability of JTC-801 to inhibit N/OFQ-stimulated [(35) S]-GTPgammaS binding was confirmed in rat brain membranes. Anxiety-like behaviour and pain sensitivity were monitored by changes in elevated plus maze performance and withdrawal responses to thermal and mechanical stimuli. Serum corticosterone and N/OFQ content in CSF, serum and brain tissues were determined by radioimmunoassay; NOP receptor protein and gene expression in amygdala, hippocampus and periaqueductal grey (PAG) were examined by immunoblotting and real-time PCR respectively. KEY RESULTS: JTC-801 treatment reversed SPS-induced mechanical allodynia, thermal hyperalgesia, anxiety-like behaviour and hypocortisolism. Elevated N/OFQ levels in serum, CSF, PAG and hippocampus at day 21 of SPS were blocked by JTC-801; daily JTC-801 treatment also reversed NOP receptor protein and mRNA up-regulation in amygdala and PAG. CONCLUSION AND IMPLICATIONS: JTC-801 reversed SPS-induced anxiety- and pain-like behaviours, and NOP receptor system up-regulation. These findings suggest that N/OFQ plays an important role in hyperalgesia and allodynia maintenance after SPS. NOP receptor antagonists may provide effective treatment for co-morbid PTSD and pain. LINKED ARTICLES: This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2.
Pharmacological profiles of a novel opioid receptor-like1 (ORL(1)) receptor antagonist, JTC-801.[Pubmed:11815367]
Br J Pharmacol. 2002 Jan;135(2):323-32.
Pharmacological effects of a novel opioid receptor-like1 (ORL(1)) receptor antagonist, [N-(4-amino-2-methylquinolin-6-yl)-2-(4-ethylphenoxymethyl) benzamide monohydrochloride] (JTC-801), were examined in in vitro and in vivo. JTC-801 inhibited the binding of [(3)H]-nociceptin to human ORL(1) receptors expressed in HeLa cells with a K(i) value of 44.5 nM. JTC-801 completely antagonized the suppression of nociceptin on forskolin-induced accumulation of cyclic AMP (IC(50) : 2.58 microM) using ORL(1) receptor expressing HeLa cells in vitro. In in vivo, when given intravenously at dosages of 0.01 mg kg(-1) and above, or orally at dosages 1 mg kg(-1) and above, JTC-801 antagonized the nociceptin-induced allodynia in mice. Effects of JTC-801 on various nociceptive models were examined. In mouse hot-plate test, JTC-801 prolonged escape response latency (ERL) to exposed heat stimulus with minimum effective doses (MED) of 0.01 mg kg(-1) by i.v. or 1 mg kg(-1) by p.o. In the rat formalin test, JTC-801 reduced both the first and second phases of the nociceptive response with MED of 0.01 mg kg(-1) by i.v. administration or 1 mg kg(-1) by p.o. administration. This anti-nociceptive action of JTC-801 was not inhibited by naloxone (10 mg kg(-1), s.c.). We have demonstrated that JTC-801 antagonizes the ORL(1) receptor response, and that JTC-801 has efficacious and potent anti-nociceptive effects in acute pain animal models not only by intravenous injection but also oral administration. These results suggest that JTC-801 may represent a new class of analgesics.
4-Aminoquinolines: novel nociceptin antagonists with analgesic activity.[Pubmed:11101358]
J Med Chem. 2000 Nov 30;43(24):4667-77.
Small-molecule nociceptin antagonists were synthesized to examine their therapeutic potential. After a 4-aminoquinoline derivative was found to bind with the human ORL(1) receptor, a series of 4-aminoquinolines and related compounds were synthesized and their binding was evaluated. Elucidation of structure-activity relationships eventually led to the optimum compounds. One of these compounds, N-(4-amino-2-methylquinolin-6-yl)-2-(4-ethylphenoxymethyl)benzamide hydrochloride (11) not only antagonized nociceptin-induced allodynia in mice but also showed analgesic effect in a hot plate test using mice and in a formalin test using rats. Its analgesic effect was not antagonized by the opioid antagonist naloxone. These results indicate that this nociceptin antagonist has the potential to become a novel type of analgesic that differs from mu-opioid agonists.


