TAK-242 S enantiomerTLR 4 signaling inhibitor CAS# 243984-10-3 |
- Skepinone-L
Catalog No.:BCC1953
CAS No.:1221485-83-1
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 243984-10-3 | SDF | Download SDF |
| PubChem ID | 11545031 | Appearance | Powder |
| Formula | C15H17ClFNO4S | M.Wt | 361.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | ethyl (6S)-6-[(2-chloro-4-fluorophenyl)sulfamoyl]cyclohexene-1-carboxylate | ||
| SMILES | CCOC(=O)C1=CCCCC1S(=O)(=O)NC2=C(C=C(C=C2)F)Cl | ||
| Standard InChIKey | LEEIJTHMHDMWLJ-AWEZNQCLSA-N | ||
| Standard InChI | InChI=1S/C15H17ClFNO4S/c1-2-22-15(19)11-5-3-4-6-14(11)23(20,21)18-13-8-7-10(17)9-12(13)16/h5,7-9,14,18H,2-4,6H2,1H3/t14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

TAK-242 S enantiomer Dilution Calculator

TAK-242 S enantiomer Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7638 mL | 13.819 mL | 27.6381 mL | 55.2761 mL | 69.0951 mL |
| 5 mM | 0.5528 mL | 2.7638 mL | 5.5276 mL | 11.0552 mL | 13.819 mL |
| 10 mM | 0.2764 mL | 1.3819 mL | 2.7638 mL | 5.5276 mL | 6.9095 mL |
| 50 mM | 0.0553 mL | 0.2764 mL | 0.5528 mL | 1.1055 mL | 1.3819 mL |
| 100 mM | 0.0276 mL | 0.1382 mL | 0.2764 mL | 0.5528 mL | 0.691 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
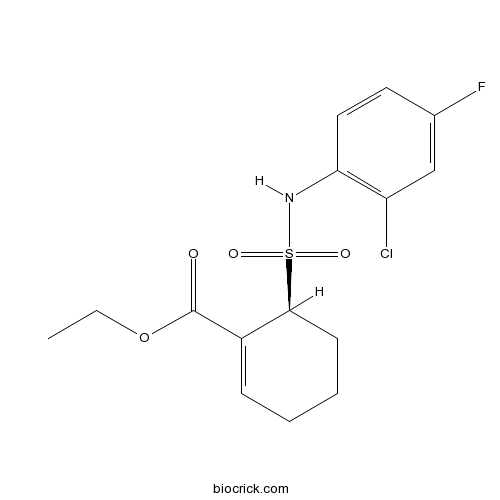
S enantiomer of TAK-242. TAK-242 (Resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling. TAK-242 is in treatment of sepsis and septic shock.
- TCS 401
Catalog No.:BCC2469
CAS No.:243967-42-2
- Cnicin
Catalog No.:BCN8546
CAS No.:24394-09-0
- Ethyl 4-methoxycinnamate
Catalog No.:BCN5028
CAS No.:24393-56-4
- Kynurenic acid sodium salt
Catalog No.:BCC7754
CAS No.:2439-02-3
- 5-Iodotubercidin
Catalog No.:BCC1312
CAS No.:24386-93-4
- Glycoside L-F2
Catalog No.:BCN2158
CAS No.:243857-99-0
- pep4c
Catalog No.:BCC5783
CAS No.:243843-43-8
- pep2m
Catalog No.:BCC5782
CAS No.:243843-42-7
- L-(-)-Fucose
Catalog No.:BCN8326
CAS No.:2438-80-4
- Bufexamac
Catalog No.:BCC4427
CAS No.:2438-72-4
- (-)-alpha-Pinene
Catalog No.:BCC8295
CAS No.:2437-95-8
- 3,5-Cycloergosta-6,8(14),22-triene
Catalog No.:BCN5100
CAS No.:24352-51-0
- TAK-242
Catalog No.:BCC1977
CAS No.:243984-11-4
- beta-D-glucose
Catalog No.:BCN8171
CAS No.:492-61-5
- (+)-Epipinoresinol
Catalog No.:BCN3255
CAS No.:24404-50-0
- Beta-Rotunol
Catalog No.:BCN6628
CAS No.:24405-57-0
- L-798,106
Catalog No.:BCC7654
CAS No.:244101-02-8
- Taxumairol R
Catalog No.:BCN6939
CAS No.:244167-04-2
- L-748,337
Catalog No.:BCC7475
CAS No.:244192-94-7
- Pulchinenoside E2
Catalog No.:BCN8186
CAS No.:244202-36-6
- Celaphanol A
Catalog No.:BCN5101
CAS No.:244204-40-8
- JTC-801
Catalog No.:BCC3800
CAS No.:244218-51-7
- LFM-A13
Catalog No.:BCC6472
CAS No.:244240-24-2
- 3,5,7,15-Tetraacetoxy-9-nicotinoyloxy-6(17),11-jatrophadien-14-one
Catalog No.:BCN6592
CAS No.:244277-75-6
Optically active cyclohexene derivative as a new antisepsis agent: an efficient synthesis of ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242).[Pubmed:16394550]
Chem Pharm Bull (Tokyo). 2006 Jan;54(1):58-62.
Two new synthetic methods were established for the efficient synthesis of optically active cyclohexene antisepsis agent, ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate [(R)-1: TAK-242)]. The first method involved recrystallization from methanol of the diastereomeric mixture (6RS,1'R)-7, obtained by esterification of carboxylic acid 3 with (S)-1-(4-nitrophenyl)ethanol [(S)-5)] to give the desired isomer (6R,1'R)-7 with 99% de in 32% yield. Subsequent catalytic hydrogenolysis and esterification gave (R)-1 with >99% ee. The second method employed enantioselective hydrolysis of acetoxymethyl ester 9a (prepared by alkylation of 3 with bromomethyl acetate) with Lipase PS-D to give the eutomeric enantiomer (R)-9a with excellent enantioselectivity (>99% ee) and high yield (48%). The desired (R)-1 was then obtained by transesterification with ethanol in the presence of concentrated sulfuric acid without loss of ee. Of these, the procedure employing enzymatic kinetic resolution using Lipase PS-D is the more efficient and practical preparation of (R)-1.


