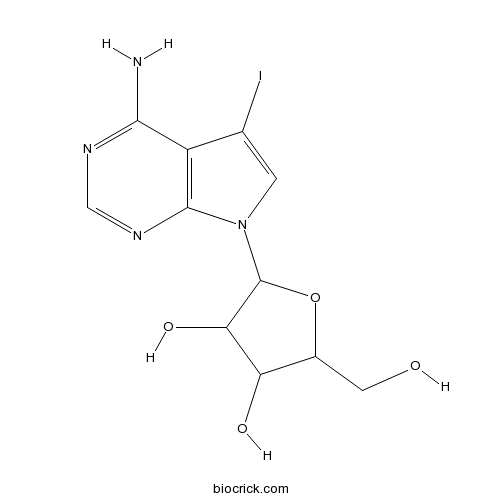
5-IodotubercidinAdenosine kinase inhibitor,potent CAS# 24386-93-4 |
- ABT 702 dihydrochloride
Catalog No.:BCC5905
CAS No.:1188890-28-9
- Pentostatin
Catalog No.:BCC1845
CAS No.:53910-25-1
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 24386-93-4 | SDF | Download SDF |
| PubChem ID | 1830 | Appearance | Powder |
| Formula | C11H13IN4O4 | M.Wt | 392.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 113939; 5-ITu | ||
| Solubility | DMSO : ≥ 49 mg/mL (124.95 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(4-amino-5-iodopyrrolo[2,3-d]pyrimidin-7-yl)-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | C1=C(C2=C(N1C3C(C(C(O3)CO)O)O)N=CN=C2N)I | ||
| Standard InChIKey | WHSIXKUPQCKWBY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H13IN4O4/c12-4-1-16(10-6(4)9(13)14-3-15-10)11-8(19)7(18)5(2-17)20-11/h1,3,5,7-8,11,17-19H,2H2,(H2,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent adenosine kinase inhibitor (IC50 = 26 nM). Also nucleoside transporter inhibitor (IC50 values are < 25 nM, 7 μM and 15 μM for inhibition of [3H]adenosine, [3H]uridine and [3H]formycin B uptake respectively). Strongly stimulates glycogen synthesis in hepatocytes via activation of glycogen synthase. Also inhibits CK1, insulin receptor tyrosine kinase, phosphorylase kinase, PKA, CK2 and PKC (IC50 values are 0.4, 3.5, 5-10, 5-10, 10.9 and 27.7 μM respectively). Decreases hippocampal DNA methylation through adenosine kinase inhibition in vivo. |

5-Iodotubercidin Dilution Calculator

5-Iodotubercidin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.55 mL | 12.7502 mL | 25.5004 mL | 51.0009 mL | 63.7511 mL |
| 5 mM | 0.51 mL | 2.55 mL | 5.1001 mL | 10.2002 mL | 12.7502 mL |
| 10 mM | 0.255 mL | 1.275 mL | 2.55 mL | 5.1001 mL | 6.3751 mL |
| 50 mM | 0.051 mL | 0.255 mL | 0.51 mL | 1.02 mL | 1.275 mL |
| 100 mM | 0.0255 mL | 0.1275 mL | 0.255 mL | 0.51 mL | 0.6375 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
5-Iodotubercidin (Itu) is a purine derivative and hence an inhibitor of adenosine kinase with an IC50 value of 26 nM [1].
Adenosine kinase is important in regulating the intracellular and extracellular concentrations of adenosine and hence diverse physiological actions of adenosine [2].
In various cells such as cancer cells, persisted AMPK activation could result in apoptosis [4]. In nude mice with colon carcinoma xenograft, Itu at a dose of 2.5 mg/kg resulted in rapid tumor regression compared with the control group. At the dose of 0.625 mg/kg, Itu still inhibited tumor growth, but p53-/- tumors were resistant to Itu at this lowered dose [1].
In male Wistar rat hepatocytes, incubation with Itu resulted in concentrations of AMP and ATP at 0.39 ± 0.06 and 1.51 ± 0.10 μmol/g cell wet mass, respectively; while control incubation at 0.27 ± 0.05 and 2.25 ± 0.33 μmol/g cell wet mass, respectively. Addition of 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) and Itu simultaneously resulted in almost the same effect of Itu alone. It was probable that Itu inhibited adenosine kinase and blocked the synthesis of 5-aminoimidazole-4-carboxamide ribonucleotide (ZMP) from AICAR. ZAM is a structural AMP analogue and hence mimics the effect of AMP on the AMP-activated protein kinase (AMPK) activation [3].
References:
[1]. Xin Zhang, Deyong Jia, Huijuan Liu, et al. Identification of 5-Iodotubercidin as a Genotoxic Drug with Anti-Cancer Potential. PLOS ONE, 2013, 8(5):e62527.
[2]. Jaoek Park and Radhey S. Gupta. Adenosine: A Key Link between Metabolism and Brain Activity: Adenosine Metabolism, Adenosine Kinase, and Evolution. New York: Springer Science+Business Media, 2013.
[3]. García-Villafranca J. and Castro J. Effects of 5-iodotubercidin on hepatic fatty acid metabolism mediated by the inhibition of acetyl-CoA carboxylase. Biochem. Pharmacol., 2002, 63(11):1997-2000.
[4]. Haiyan Chen, Ji-ping Wang, Richard J. Santen, et al. Adenosine monophosphate activated protein kinase (AMPK), a mediator of estradiol-induced apoptosis in long-term estrogen deprived breast cancer cells. Apoptosis, 2015, 20:821-830.
- Glycoside L-F2
Catalog No.:BCN2158
CAS No.:243857-99-0
- pep4c
Catalog No.:BCC5783
CAS No.:243843-43-8
- pep2m
Catalog No.:BCC5782
CAS No.:243843-42-7
- L-(-)-Fucose
Catalog No.:BCN8326
CAS No.:2438-80-4
- Bufexamac
Catalog No.:BCC4427
CAS No.:2438-72-4
- (-)-alpha-Pinene
Catalog No.:BCC8295
CAS No.:2437-95-8
- 3,5-Cycloergosta-6,8(14),22-triene
Catalog No.:BCN5100
CAS No.:24352-51-0
- S-(5'-Adenosyl)-L-methionine chloride
Catalog No.:BCN2229
CAS No.:24346-00-7
- Apamin
Catalog No.:BCC7141
CAS No.:24345-16-2
- 6-Amino-1-methyluracil
Catalog No.:BCC8757
CAS No.:2434-53-9
- Nagilactone C
Catalog No.:BCN4040
CAS No.:24338-53-2
- Tricosanoic acid
Catalog No.:BCN5394
CAS No.:2433-96-7
- Kynurenic acid sodium salt
Catalog No.:BCC7754
CAS No.:2439-02-3
- Ethyl 4-methoxycinnamate
Catalog No.:BCN5028
CAS No.:24393-56-4
- Cnicin
Catalog No.:BCN8546
CAS No.:24394-09-0
- TCS 401
Catalog No.:BCC2469
CAS No.:243967-42-2
- TAK-242 S enantiomer
Catalog No.:BCC1978
CAS No.:243984-10-3
- TAK-242
Catalog No.:BCC1977
CAS No.:243984-11-4
- beta-D-glucose
Catalog No.:BCN8171
CAS No.:492-61-5
- (+)-Epipinoresinol
Catalog No.:BCN3255
CAS No.:24404-50-0
- Beta-Rotunol
Catalog No.:BCN6628
CAS No.:24405-57-0
- L-798,106
Catalog No.:BCC7654
CAS No.:244101-02-8
- Taxumairol R
Catalog No.:BCN6939
CAS No.:244167-04-2
- L-748,337
Catalog No.:BCC7475
CAS No.:244192-94-7
Slowly on, Slowly off: Bisubstrate-Analogue Conjugates of 5-Iodotubercidin and Histone H3 Peptide Targeting Protein Kinase Haspin.[Pubmed:28181383]
Chembiochem. 2017 Apr 18;18(8):790-798.
The atypical protein kinase haspin is a key player in mitosis by catalysing the phosphorylation of Thr3 in histone H3, and thus ensuring the normal function of the chromosomal passenger complex. Here, we report the development of bisubstrate-analogue inhibitors targeting haspin. The compounds were constructed by linking 5-Iodotubercidin to the N terminus of histone H3 peptide. The new conjugates show high affinity (sub-nanomolar KD ) towards haspin as well as slow kinetics of association and dissociation (residence time of several hours). This reflects a unique binding mode and translated into improved selectivity. The latter was confirmed in a biochemical binding/displacement assay with a panel of ten protein kinases, in a thermal shift assay with off-targets of 5-Iodotubercidin (adenosine kinase and the Cdc2-like kinase family) and in assay with spiked HeLa cell lysate.
Identification of 5-Iodotubercidin as a genotoxic drug with anti-cancer potential.[Pubmed:23667485]
PLoS One. 2013 May 7;8(5):e62527.
Tumor suppressor p53, which is activated by various stress and oncogene activation, is a target for anti-cancer drug development. In this study, by screening panels of protein kinase inhibitors and protein phosphatase inhibitors, we identified 5-Iodotubercidin as a strong p53 activator. 5-Iodotubercidin is purine derivative and is used as an inhibitor for various kinases including adenosine kinase. We found that 5-Iodotubercidin could cause DNA damage, verified by induction of DNA breaks and nuclear foci positive for gammaH2AX and TopBP1, activation of Atm and Chk2, and S15 phosphorylation and up-regulation of p53. As such, 5-Iodotubercidin induces G2 cell cycle arrest in a p53-dependent manner. Itu also induces cell death in p53-dependent and -independent manners. DNA breaks were likely generated by incorporation of 5-Iodotubercidin metabolite into DNA. Moreover, 5-Iodotubercidin showed anti-tumor activity as it could reduce the tumor size in carcinoma xenograft mouse models in p53-dependent and -independent manners. These findings reveal 5-Iodotubercidin as a novel genotoxic drug that has chemotherapeutic potential.
Effects of 5-iodotubercidin on hepatic fatty acid metabolism mediated by the inhibition of acetyl-CoA carboxylase.[Pubmed:12093476]
Biochem Pharmacol. 2002 Jun 1;63(11):1997-2000.
Diverse mechanisms of action have been proposed for 5-Iodotubercidin, although it is widely used as an adenosine kinase inhibitor that consequently interferes with the metabolism of adenosine and adenine nucleotides. Incubation of rat hepatocytes with iodotubercidin produced important effects on lipid metabolism. (i) Both acetyl-CoA carboxylase and fatty acid synthesis de novo were inhibited in parallel by iodotubercidin, with no change in the activity of fatty acid synthase. The inhibition of both activities showed a comparable dependence on iodotubercidin concentration and was accompanied by a similar decrease (about 60%) in the intracellular malonyl-CoA concentration. (ii) Iodotubercidin stimulated palmitate oxidation, although octanoate oxidation was unaffected. However, this effect can be attributed to the decrease of malonyl-CoA concentration and the concomitant relief of the inhibition of carnitine palmitoyltransferase I, because the activity of this enzyme was found unaltered when determined in cells permeabilized with digitonin. (iii) Iodotubercidin also inhibited cholesterol synthesis de novo. Results, thus, indicate that iodotubercidin increases fatty acid oxidation activity of the liver at the expense of lipogenesis, and we suggest that these effects on fatty acid metabolism are mediated by the inhibition of acetyl-CoA carboxylase, probably due to a more than twice increase in the AMP/ATP ratio and the concomitant stimulation of the AMP-activated protein kinase.
Threonine phosphorylation of integrin beta3 in calyculin A-treated platelets is selectively sensitive to 5'-iodotubercidin.[Pubmed:17052767]
Biochim Biophys Acta. 2007 Feb;1773(2):185-91.
Exposure of platelets to toxins (calyculin A or okadaic acid) that inhibit protein serine/threonine phosphatases types 1 and 2A, at concentrations that block aggregatory and secretory responses, results in the phosphorylation of several platelet proteins including integrin beta(3). Since protein phosphorylation represents a balance between kinase and phosphatase activities, this increase in phosphorylation reflects either the removal of phosphatases that oppose constitutively active kinases known to reside in the platelet (e.g., casein kinase 2) or the activation of endogenous kinases. In this study, we demonstrate that the addition of calyculin A promotes the activation of several endogenous platelet protein kinases, including p42/44(mapk), p38(mapk), Akt/PKB, and LKB1. Using a pharmacologic approach, we assessed whether inhibition of these and other enzymes block phosphorylation of beta(3). Inhibitors of p38(mapk), casein kinase, AMP kinase, protein kinase C, and calcium-calmodulin-dependent kinases did not block phosphorylation of beta(3) on thr(753). In contrast, 5'-iodotubercidin, at 50 muM, blocks beta(3) phosphorylation without affecting the efficacy of calyculin A to inhibit platelet aggregation and spreading. These data dissociate threonine phosphorylation of beta(3) molecules and inhibition of platelet responses by protein phosphatase inhibitors.
Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis.[Pubmed:23863710]
J Clin Invest. 2013 Aug;123(8):3552-63.
Epigenetic modifications, including changes in DNA methylation, lead to altered gene expression and thus may underlie epileptogenesis via induction of permanent changes in neuronal excitability. Therapies that could inhibit or reverse these changes may be highly effective in halting disease progression. Here we identify an epigenetic function of the brain's endogenous anticonvulsant adenosine, showing that this compound induces hypomethylation of DNA via biochemical interference with the transmethylation pathway. We show that inhibition of DNA methylation inhibited epileptogenesis in multiple seizure models. Using a rat model of temporal lobe epilepsy, we identified an increase in hippocampal DNA methylation, which correlates with increased DNA methyltransferase activity, disruption of adenosine homeostasis, and spontaneous recurrent seizures. Finally, we used bioengineered silk implants to deliver a defined dose of adenosine over 10 days to the brains of epileptic rats. This transient therapeutic intervention reversed the DNA hypermethylation seen in the epileptic brain, inhibited sprouting of mossy fibers in the hippocampus, and prevented the progression of epilepsy for at least 3 months. These data demonstrate that pathological changes in DNA methylation homeostasis may underlie epileptogenesis and reversal of these epigenetic changes with adenosine augmentation therapy may halt disease progression.
Adenosine kinase inhibitors. 1. Synthesis, enzyme inhibition, and antiseizure activity of 5-iodotubercidin analogues.[Pubmed:10956196]
J Med Chem. 2000 Jul 27;43(15):2883-93.
Adenosine receptor agonists produce a wide variety of therapeutically useful pharmacologies. However, to date they have failed to undergo successful clinical development due to dose-limiting side effects. Adenosine kinase inhibitors (AKIs) represent an alternative strategy, since AKIs may raise local adenosine levels in a more site- and event-specific manner and thereby elicit the desired pharmacology with a greater therapeutic window. Starting with 5-Iodotubercidin (IC50 = 0.026 microM) and 5'-amino-5'-deoxyadenosine (IC50 = 0.17 microM) as lead inhibitors of the isolated human AK, a variety of pyrrolo[2,3-d]pyrimidine nucleoside analogues were designed and prepared by coupling 5-substituted-4-chloropyrrolo[2,3-d]pyrimidine bases with ribose analogues using the sodium salt-mediated glycosylation procedure. 5'-Amino-5'-deoxy analogues of 5-bromo- and 5-Iodotubercidins were found to be the most potent AKIs reported to date (IC50S < 0.001 microM). Several potent AKIs were shown to exhibit anticonvulsant activity in the rat maximal electric shock (MES) induced seizure assay.
Effects of iodotubercidin on adenosine kinase activity and nucleoside transport in DDT1 MF-2 smooth muscle cells.[Pubmed:8667202]
J Pharmacol Exp Ther. 1996 Jun;277(3):1397-401.
Iodotubercidin is an adenosine kinase inhibitor that through its ability to increase levels of endogenous adenosine can enhance adenosine's receptor-mediated effects. We investigated whether iodotubercidin can inhibit [3H]adenosine accumulation by inhibiting transport processes in addition to inhibition of intracellular trapping of labeled adenine nucleotides. Under conditions in which extensive metabolism of intracellular adenosine was present, [3H]adenosine accumulation by DDT1 MF-2 cells was almost completely inhibited by iodotubercidin and the adenosine deaminase inhibitor erythro-9-(2-hydroxy-3-nonyl)-adenine or by the nucleoside transport inhibitor nitrobenzylthioinosine. By using similar conditions, [3H]adenosine accumulation was significantly greater in Na+ buffer than in buffer containing N-methyl-D-glucamine in place of Na+; however, this effect may be explained by an observed 40% inhibition of adenosine kinase activity by N-methyl-D-glucamine. By using uptake intervals of 14 sec to represent the transport component of uptake, iodotubercidin decreased the affinity for adenosine, by about 3-fold, but had no effect on maximum velocity of transport. That these effects of iodotubercidin were due to direct interactions with nucleoside transporters was supported by findings that iodotubercidin inhibited [3H]nitrobenzylthioinosine binding to nucleoside transporters with a Ki value of 4 microM and inhibited [3H]uridine and [3H]formycin B uptake with IC50 values of 7 and 15 microM, respectively. These data suggest that iodotubercidin, at pharmacologically relevant concentrations, inhibits nucleoside transport independently of its well characterized inhibition of adenosine kinase and that N-methyl-D-glucamine must be used with caution in experiments to determine the possible presence of Na+ gradient-dependent concentrative nucleoside transporters.
Identification of the glycogenic compound 5-iodotubercidin as a general protein kinase inhibitor.[Pubmed:8166629]
Biochem J. 1994 Apr 1;299 ( Pt 1):123-8.
Addition of micromolar concentrations of the adenosine derivative 5-Iodotubercidin (Itu) initiates glycogen synthesis in isolated hepatocytes by causing inactivation of phosphorylase and activation of glycogen synthase [Fluckiger-Isler and Walter (1993) Biochem. J. 292, 85-91]. We report here that Itu also antagonizes the effects of saturating concentrations of glucagon and vasopressin on these enzymes. The Itu-induced activation of glycogen synthase could not be explained by the removal of phosphorylase a (a potent inhibitor of the glycogen-associated synthase phosphatase). When tested on purified enzymes, Itu did not affect the activities of the major Ser/Thr-specific protein phosphatases (PP-1, PP-2A, PP-2B and PP-2C), but it inhibited various Ser/Thr-specific protein kinases as well as the tyrosine kinase activity of the insulin receptor (IC50 between 0.4 and 28 microM at 10-15 microM ATP). Tubercidin, which did not affect glycogen synthase or phosphorylase in liver cells, was 300 times less potent as a protein kinase inhibitor. Kinetic analysis of the inhibition of casein kinase-1 and protein kinase A showed that Itu acts as a competitive inhibitor with respect to ATP, and as a mixed-type inhibitor with respect to the protein substrate. We propose that Itu inactivates phosphorylase and activates glycogen synthase by inhibiting phosphorylase kinase and various glycogen synthase kinases. Consistent with the broad specificity of Itu in vitro, this compound decreased the phosphorylation level of numerous phosphopolypeptides in intact liver cells. Our data suggest that at least some of the biological effects of Itu can be explained by an inhibition of protein kinases.


