RBC8Ral GTPase inhibitor CAS# 361185-42-4 |
- Safinamide Mesylate
Catalog No.:BCC2320
CAS No.:202825-46-5
- Moclobemide (Ro 111163)
Catalog No.:BCC2322
CAS No.:71320-77-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

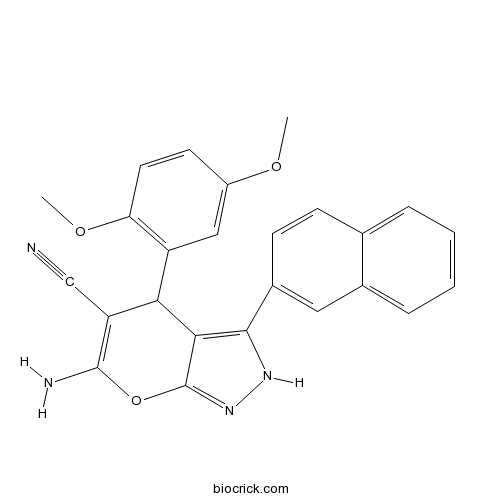
| Cas No. | 361185-42-4 | SDF | Download SDF |
| PubChem ID | 6626226 | Appearance | Powder |
| Formula | C25H20N4O3 | M.Wt | 424.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 40 mg/mL (94.24 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 6-amino-4-(2,5-dimethoxyphenyl)-3-naphthalen-2-yl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile | ||
| SMILES | COC1=CC(=C(C=C1)OC)C2C(=C(OC3=NNC(=C23)C4=CC5=CC=CC=C5C=C4)N)C#N | ||
| Standard InChIKey | CLMQBVUFKIKYLU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H20N4O3/c1-30-17-9-10-20(31-2)18(12-17)21-19(13-26)24(27)32-25-22(21)23(28-29-25)16-8-7-14-5-3-4-6-15(14)11-16/h3-12,21H,27H2,1-2H3,(H,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | RalA and RalB GTPase inhibitor (EC50 ~3.5 μM). Inhibits the binding of Ral to RaLBP1. Exhibits no detectable inhibition of Ras or RhoA activity. Suppresses growth of xenograft tumors in mice. Cell permeable. |

RBC8 Dilution Calculator

RBC8 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.356 mL | 11.78 mL | 23.5599 mL | 47.1198 mL | 58.8998 mL |
| 5 mM | 0.4712 mL | 2.356 mL | 4.712 mL | 9.424 mL | 11.78 mL |
| 10 mM | 0.2356 mL | 1.178 mL | 2.356 mL | 4.712 mL | 5.89 mL |
| 50 mM | 0.0471 mL | 0.2356 mL | 0.4712 mL | 0.9424 mL | 1.178 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2356 mL | 0.4712 mL | 0.589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
RBC8 inhibit Ral GTPase with IC50 values of 3.5 μM and 3.4 μM in Ral-dependent lines H2122 and H358 [1].
RalA and B are Ras-like GTPases. They are important drivers of metastasis and tumor growth [1].
RBC8 reduced the activation of RalA in living cells. Ral is required for spreading murine embryonic fibroblasts (MEFs) and lipid raft exocytosis on fibronectin-coated cover slips. In these cells, the spreading of WT MEFs was inhibited by the depletion of RalA via siRNA, whereas caveolin deficient (Cav1-/-) MEFs were resistant to RalA depletion. Treatment with RBC8 inhibited only the cell spreading in the WT MEFs, it did not inhibited the cell spreading in Cav1-/- MEFs. A Ral pull-down assay showed that RBC8 inhibited the activation of both RalA and RalB in both the H2122 and H358 cell lines. In H2122 and H358 cells with Ral knockdown by siRNA, treatment with RBC8 did not show further inhibition of colony formation [1].
In nude mice inoculated with H2122 human lung cancer cells subcutaneously, treatment with RBC8 at 50 mg/kg/d for 21 days (except weekends) intraperitoneally showed an inhibitory effect on tumor growth to a similar extent as dual knockdown of RalA and B. H358 is a lung cancer line. In this cell line, similar results were yielded [1].
Reference:
[1]. Yan C, Liu D, Li L, et al. Discovery and characterization of small molecules that target the Ral GTPase. Nature, 2014, 515(7527): 443-447.
- Propranolol glycol
Catalog No.:BCC6817
CAS No.:36112-95-5
- Sodium cholate
Catalog No.:BCN6981
CAS No.:361-09-1
- B-HT 920
Catalog No.:BCC1417
CAS No.:36085-73-1
- B-HT 933 dihydrochloride
Catalog No.:BCC7474
CAS No.:36067-72-8
- Octahydrocurcumin
Catalog No.:BCN2725
CAS No.:36062-07-4
- Hexahydrocurcumin
Catalog No.:BCN4641
CAS No.:36062-05-2
- Tetrahydrocurcumin
Catalog No.:BCN2724
CAS No.:36062-04-1
- Dihydrosanguinarine
Catalog No.:BCN3713
CAS No.:3606-45-9
- Alpinetin
Catalog No.:BCN5315
CAS No.:36052-37-6
- Diepiserratenediol
Catalog No.:BCN7433
CAS No.:3604-92-0
- Ecdysone
Catalog No.:BCN2629
CAS No.:3604-87-3
- 2',4'-Dihydroxy-6'-methoxyacetophenone
Catalog No.:BCN5314
CAS No.:3602-54-8
- 3,4-Secolupa-4(23),20(29)-diene-3,28-dioic acid
Catalog No.:BCN7243
CAS No.:36138-41-7
- Saxagliptin
Catalog No.:BCC3934
CAS No.:361442-04-8
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- D-(+)-Fucose
Catalog No.:BCN6432
CAS No.:3615-37-0
- alpha-L-Rhamnose
Catalog No.:BCN2592
CAS No.:3615-41-6
- Phytin
Catalog No.:BCN1285
CAS No.:3615-82-5
- Mullilam diol
Catalog No.:BCN5316
CAS No.:36150-04-6
- Saxalin
Catalog No.:BCC8357
CAS No.:36150-06-8
- Dehydroleucodine
Catalog No.:BCN6897
CAS No.:36150-07-9
- Kobusin
Catalog No.:BCN7563
CAS No.:36150-23-9
- Blumenol B
Catalog No.:BCN5317
CAS No.:36151-01-6
- 3-Acetyl-2,5-dichlorothiophene
Catalog No.:BCC8602
CAS No.:36157-40-1
Characterisation of the Ral GTPase inhibitor RBC8 in human and mouse platelets.[Pubmed:30880223]
Cell Signal. 2019 Mar 14;59:34-40.
The Ral GTPases, RalA and RalB, have been implicated in numerous cellular processes, but are most widely known for having regulatory roles in exocytosis. Recently, we demonstrated that deletion of both Ral genes in a platelet-specific mouse gene knockout caused a substantial defect in surface exposure of P-selectin, with only a relatively weak defect in platelet dense granule secretion that did not alter platelet functional responses such as aggregation or thrombus formation. We sought to investigate the function of Rals in human platelets using the recently described Ral inhibitor, RBC8. Initial studies in human platelets confirmed that RBC8 could effectively inhibit Ral GTPase activation, with an IC50 of 2.2muM and 2.3muM for RalA and RalB, respectively. Functional studies using RBC8 revealed significant, dose-dependent inhibition of platelet aggregation, secretion (alpha- and dense granule), integrin activation and thrombus formation, while alpha-granule release of platelet factor 4, Ca(2+) signalling or phosphatidylserine exposure were unaltered. Subsequent studies in RalAB-null mouse platelets pretreated with RBC8 showed dose-dependent decreases in integrin activation and dense granule secretion, with significant inhibition of platelet aggregation and P-selectin exposure at 10muM RBC8. This study strongly suggests therefore that although RBC8 is useful as a Ral inhibitor in platelets, it is likely also to have off-target effects in the same concentration range as for Ral inhibition. So, whilst clearly useful as a Ral inhibitor, interpretation of data needs to take this into account when assessing roles for Rals using RBC8.
Two-Step Forward Genetic Screen in Mice Identifies Ral GTPase-Activating Proteins as Suppressors of Hepatocellular Carcinoma.[Pubmed:27178121]
Gastroenterology. 2016 Aug;151(2):324-337.e12.
BACKGROUND & AIMS: High-throughput sequencing technologies have identified thousands of infrequently mutated genes in hepatocellular carcinomas (HCCs). However, high intratumor and intertumor heterogeneity, combined with large numbers of passenger mutations, have made it difficult to identify driver mutations that contribute to the development of HCC. We combined transposon mutagenesis with a high-throughput screen of a small-hairpin RNA (shRNA) library to identify genes and pathways that contribute to HCC development. METHODS: Sleeping beauty transposons were mobilized in livers of transgenic mice predisposed to develop hepatocellular adenoma and HCC owing to expression of the hepatitis B virus surface antigen. This whole-genome mutagenesis technique was used to generate an unbiased catalogue of candidate cancer genes (CCGs). Pooled shRNA libraries targeting 250 selected CCGs then were introduced into immortalized mouse liver cells and the cells were monitored for their tumor-forming ability after injection into nude mice. RESULTS: Transposon-mediated mutagenesis identified 1917 high-confident CCGs and highlighted the importance of Ras signaling in the development of HCC. Subsequent pooled shRNA library screening of 250 selected CCGs validated 27 HCC tumor-suppressor genes. Individual shRNA knockdown of 4 of these genes (Acaa2, Hbs1l, Ralgapa2, and Ubr2) increased the proliferation of multiple human HCC cell lines in culture and accelerated the formation of xenograft tumors in nude mice. The ability of Ralgapa2 to promote HCC cell proliferation and tumor formation required its inhibition of Rala and Ralb. Dual inhibition of Ras signaling via Ral and Raf, using a combination of small-molecule inhibitor RBC8 and sorafenib, reduced the proliferation of HCC cells in culture and completely inhibited their growth as xenograft tumors in nude mice. CONCLUSIONS: In a 2-step forward genetic screen in mice, we identified members of the Ral guanosine triphosphatase-activating protein pathway and other proteins as suppressors of HCC cell proliferation and tumor growth. These proteins might serve as therapeutic targets for liver cancer.
RalA, a GTPase targeted by miR-181a, promotes transformation and progression by activating the Ras-related signaling pathway in chronic myelogenous leukemia.[Pubmed:26967392]
Oncotarget. 2016 Apr 12;7(15):20561-73.
BCR/ABL is a well-known activator of multiple signaling pathways. RalA, a Ras downstream signaling molecule and a small GTPase, plays an important role in Bcr-Abl-induced leukemogenesis but the exact mechanism remains elusive. Here, we show that RalA GTPase activity is commonly high in chronic myelogenous leukemia (CML) cell lines and patient samples. Overexpression of RalA results in malignant transformation and progression, and induces resistance to imatinib (IM) in BaF3 and K562 cell lines. RalA reduced survival and led to IM resistance in a xenografted mouse model. Ablation of RalA by either siRNA or miR-181a, a RalA targeting microRNA, attenuated the malignant phenotypes in K562 cells. RBC8, a selective Ral inhibitor, enhanced the inhibitory effects of IM in K562, KCL22 and BaF3-P210 cells. Interestingly, the phospho-specific protein microarray assay revealed that multiple phosphorylation signal proteins were decreased by RalA inhibition, including SAPK, JNK, SRC, VEGFR2, P38 MAPK, c-Kit, JunB, and Keratin18. Among them, P38 MAPK and SAPK/JNK are Ras downstream signaling kinases. Taken together, RalA GTPase might be an important oncogene activating the Ras-related signaling pathway in CML.
Discovery and characterization of small molecules that target the GTPase Ral.[Pubmed:25219851]
Nature. 2014 Nov 20;515(7527):443-7.
The Ras-like GTPases RalA and RalB are important drivers of tumour growth and metastasis. Chemicals that block Ral function would be valuable as research tools and for cancer therapeutics. Here we used protein structure analysis and virtual screening to identify drug-like molecules that bind to a site on the GDP-bound form of Ral. The compounds RBC6, RBC8 and RBC10 inhibited the binding of Ral to its effector RALBP1, as well as inhibiting Ral-mediated cell spreading of murine embryonic fibroblasts and anchorage-independent growth of human cancer cell lines. The binding of the RBC8 derivative BQU57 to RalB was confirmed by isothermal titration calorimetry, surface plasmon resonance and (1)H-(15)N transverse relaxation-optimized spectroscopy (TROSY) NMR spectroscopy. RBC8 and BQU57 show selectivity for Ral relative to the GTPases Ras and RhoA and inhibit tumour xenograft growth to a similar extent to the depletion of Ral using RNA interference. Our results show the utility of structure-based discovery for the development of therapeutics for Ral-dependent cancers.


