Blumenol BCAS# 36151-01-6 |
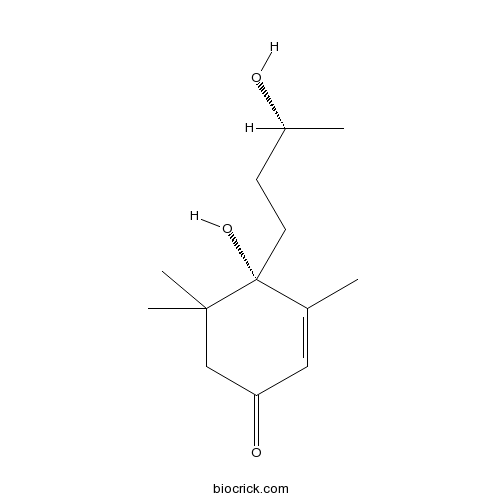
- 9-Epiblumenol B
Catalog No.:BCN5075
CAS No.:22841-42-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 36151-01-6 | SDF | Download SDF |
| PubChem ID | 14135402 | Appearance | Oil |
| Formula | C13H22O3 | M.Wt | 226.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4S)-4-hydroxy-4-[(3R)-3-hydroxybutyl]-3,5,5-trimethylcyclohex-2-en-1-one | ||
| SMILES | CC1=CC(=O)CC(C1(CCC(C)O)O)(C)C | ||
| Standard InChIKey | CWOFGGNDZOPNFG-ZWNOBZJWSA-N | ||
| Standard InChI | InChI=1S/C13H22O3/c1-9-7-11(15)8-12(3,4)13(9,16)6-5-10(2)14/h7,10,14,16H,5-6,8H2,1-4H3/t10-,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Química Nova 12/2010; 34(7):1182-1187.Chemical constituents and Leishmanicidal activity of Gustavia elliptica (Lecythidaceae)[Reference: WebLink]
|

Blumenol B Dilution Calculator

Blumenol B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4189 mL | 22.0946 mL | 44.1891 mL | 88.3783 mL | 110.4728 mL |
| 5 mM | 0.8838 mL | 4.4189 mL | 8.8378 mL | 17.6757 mL | 22.0946 mL |
| 10 mM | 0.4419 mL | 2.2095 mL | 4.4189 mL | 8.8378 mL | 11.0473 mL |
| 50 mM | 0.0884 mL | 0.4419 mL | 0.8838 mL | 1.7676 mL | 2.2095 mL |
| 100 mM | 0.0442 mL | 0.2209 mL | 0.4419 mL | 0.8838 mL | 1.1047 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kobusin
Catalog No.:BCN7563
CAS No.:36150-23-9
- Dehydroleucodine
Catalog No.:BCN6897
CAS No.:36150-07-9
- Saxalin
Catalog No.:BCC8357
CAS No.:36150-06-8
- Mullilam diol
Catalog No.:BCN5316
CAS No.:36150-04-6
- Phytin
Catalog No.:BCN1285
CAS No.:3615-82-5
- alpha-L-Rhamnose
Catalog No.:BCN2592
CAS No.:3615-41-6
- D-(+)-Fucose
Catalog No.:BCN6432
CAS No.:3615-37-0
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- Saxagliptin
Catalog No.:BCC3934
CAS No.:361442-04-8
- 3,4-Secolupa-4(23),20(29)-diene-3,28-dioic acid
Catalog No.:BCN7243
CAS No.:36138-41-7
- RBC8
Catalog No.:BCC5569
CAS No.:361185-42-4
- Propranolol glycol
Catalog No.:BCC6817
CAS No.:36112-95-5
- 3-Acetyl-2,5-dichlorothiophene
Catalog No.:BCC8602
CAS No.:36157-40-1
- 3'-O-Methylorobol
Catalog No.:BCN5318
CAS No.:36190-95-1
- Genistein 7,4'-di-O-beta-D-glucopyranoside
Catalog No.:BCN7835
CAS No.:36190-98-4
- 2-Methoxyestradiol (2-MeOE2)
Catalog No.:BCC2228
CAS No.:362-07-2
- NVP 231
Catalog No.:BCC4244
CAS No.:362003-83-6
- Columbamine
Catalog No.:BCN2722
CAS No.:3621-36-1
- Jatrorrhizine
Catalog No.:BCN5319
CAS No.:3621-38-3
- Cyclo(D-Leu-L-Pro)
Catalog No.:BCN4028
CAS No.:36238-67-2
- TC 1
Catalog No.:BCC7450
CAS No.:362512-81-0
- (±)-SLV 319
Catalog No.:BCC7954
CAS No.:362519-49-1
- Clemaphenol A
Catalog No.:BCN7834
CAS No.:362606-60-8
- Pitolisant
Catalog No.:BCC1862
CAS No.:362665-56-3
[Chemical constitutes from Clerodendrum japonicum].[Pubmed:30111024]
Zhongguo Zhong Yao Za Zhi. 2018 Jul;43(13):2732-2739.
The chemical constituents from the ethanol extract of Clerodendrum japonicum were isolated by a combination of various chromatographic techniques including column chromatography over silica gel, sephadex LH-20, ODS and reversed phase HPLC. Sixteen compounds with a pair of epimers were elucidated through the application of physicochemical properties with modern spectral analysis technology as 7alpha-hydroxy syringaresinol (1), (-)-syringaresinol (2), (-)-medioresinol (3), 2'',3''-O-acetylmartyonside (4), 2''-O-acetyl-martyonside (5), martinoside (6), monoacetyl martinoside (7)cytochalasin O (8), 9-epi-Blumenol B (9), (6R, 9S) and (6R,9R)-9-hydroxy-4-megastigmen-3-one (10a10b), (6R,9S)-3-oxo-alpha-ionol (11), (-)-dehydrovomifoliol (12)megastigm-5-en-3,9-diol (13), (3R,6E,10S)-2,6,10-trimethyl-3-hydroxydodeca-6,11-diene-2,10-diol (14), (2R)-butylitaconic acid (15), 3-(3&-hydroxybutyl)-2,4,4-trimethylcyclohexa-2,5-dienone (16), (-)-loliolide (17), of which compound 1 and 15 are new natural product, the other compounds were isolated for the first time from Clerodendrum japonicum except for compounds 4, 6 and 7.
Chemical Constituents of the Ethyl Acetate Extract from Diaphragma juglandis Fructus and Their Inhibitory Activity on Nitric Oxide Production In Vitro.[Pubmed:29286331]
Molecules. 2017 Dec 29;23(1). pii: molecules23010072.
Diaphragma juglandis fructus contains various bioactive constituents. Fourteen compounds were isolated from Diaphragma juglandis fructus by preparative high performance liquid chromatography (pre-HPLC) and high-speed counter-current chromatography (HSCCC). Their structures were identified by nuclear magnetic resonance (NMR) and electrospray ionization mass spectrometry (ESI-MS). Compounds (+)-dehydrovomifoliol (12), (6R,9R)-9-hydroxymegastigman-4-en-3-one (13) and (6R,9S)-9-hydroxymegastigman-4-en-3-one (14) are found from Juglans regia L. for the first time. Compounds dihydrophaseic acid (2), Blumenol B (3) and (4S)-4-hydroxy-1-tetralone (11) are isolated from Diaphragma juglandis fructus for the first time. The anti-inflammatory effects of isolated compounds were evaluated by an in vitro model of lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Compounds gallic acid (1), ethyl gallate (9) and (+)-dehydrovomifoliol (12) exhibited inhibitory activity on the nitric oxide production of RAW 264.7 at a concentration of 25 muM. The result indicated that the combination HSCCC with pre-HPLC is an effective way for compound separation and purification. And Diaphragma juglandis fructus constituents have the potential for the treatment of inflammatory-related diseases.
Two new C(13) nor-isoprenoids from the leaves of Casearia sylvestris.[Pubmed:19483351]
Chem Pharm Bull (Tokyo). 2009 Jun;57(6):636-8.
Two new C(13) nor-isoprene glycosides, (6S,9S)-6,9-dihydroxymegastiman-4-en-9-O-beta-D-glucopyranoside (1) and (6S,9S)-6,9-dihydroxymegastiman-4-en-9-O-beta-D-apiofuranosyl-(1-->6)-beta-D-gluc opyranoside (2) were isolated from the leaves of Casearia sylvestris, along with icariside B(5) (3), byzantionoside B (4), Blumenol B (5), blumenol C (6) and loliolide (7). The structures of these compounds were determined on the basis of 1D and 2D NMR, MS and circular dichroism (CD) spectroscopic analyses, chemical methods and comparison with the literature data.
[Studies on chemical constituents of aerial parts of Ammopiptanthus mongolicus].[Pubmed:19166007]
Zhongguo Zhong Yao Za Zhi. 2008 Oct;33(19):2204-6.
OBJECTIVE: To investigate the chemical constituents of the aerial parts of Ammopiptanthus mongolicus. METHOD: The chemical constituents were isolated by various column chromatographic methods. The structures were identified by spectral data. RESULT: Ten compounds were isolated and identified as m-hydroxybenzoic acid (1), 1-(4-hydroxyphenyl) ethanone (2), beta-sitosterol (3), (-)-syringaresinol (4), (+)-lariciresinol (5), blumenol A (6), Blumenol B (7), beta-daucosterol (8), coniferin (9), syringin (10). CONCLUSION: The ten compounds were obtained from the genus Ammopiptanthus for the first time.
Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity.[Pubmed:18596648]
Molecules. 2008 Jun 1;13(6):1219-29.
Dragon's blood (Sangre de drago), a viscous red sap derived from Croton lechleri Muell-Arg (Euphorbiaceae), is extensively used by indigenous cultures of the Amazonian basin for its wound healing properties. The aim of this study was to identify the minor secondary metabolites and test the antioxidant activity of this sustance. A bioguided fractionation of the n-hexane, chloroform, n-butanol, and aqueous extracts led to the isolation of 15 compounds: three megastigmanes, four flavan-3-ols, three phenylpropanoids, three lignans, a clerodane, and the alkaloid taspine. In addition to these known molecules, six compounds were isolated and identified for the first time in the latex: Blumenol B, blumenol C, 4,5-dihydroblumenol A, erythro-guaiacyl-glyceryl-beta-O-4'- dihydroconiferyl ether, 2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-propane-1,3-diol and floribundic acid glucoside. Combinations of spectroscopic methods ((1)H-, (13)C- NMR and 2D-NMR experiments), ESI-MS, and literature comparisons were used for compound identification. In vitro antioxidant activities were assessed by DPPH, total antioxidant capacity and lipid peroxidation assays. Flavan-3-ols derivatives (as major phenolic compounds in the latex) exhibited the highest antioxidant activity.


