TC 1CAS# 362512-81-0 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

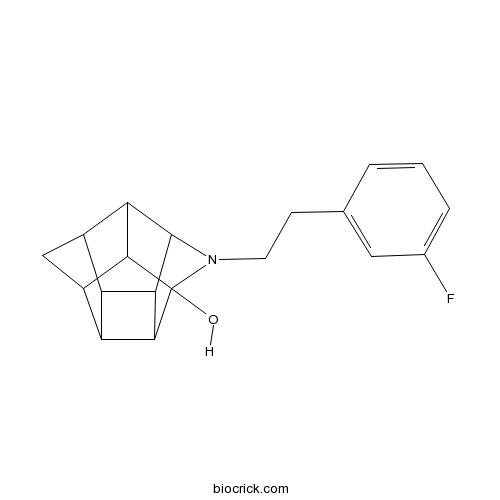
| Cas No. | 362512-81-0 | SDF | Download SDF |
| PubChem ID | 10040286 | Appearance | Powder |
| Formula | C19H20FNO | M.Wt | 297.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO with gentle warming and to 50 mM in ethanol with gentle warming | ||
| SMILES | C1C2C3C4C1C5C2C6C3C4C5(N6CCC7=CC(=CC=C7)F)O | ||
| Standard InChIKey | FYGREZKTJIXWIH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20FNO/c20-9-3-1-2-8(6-9)4-5-21-18-14-10-7-11-13-12(10)15(18)17(13)19(21,22)16(11)14/h1-3,6,10-18,22H,4-5,7H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity σ1 receptor ligand that displays selectivity over σ2 receptors (Ki values are 10 and 370 nM respectively). Exhibits low affinity for dopamine (DAT), serotonin (SERT) and noradrenalin (NET) transporters (Ki > 10 μM) and low affinity for dopamine D2 receptors (Ki = 1226 nM). Attenuates cocaine-induced locomotor activity in mice. |

TC 1 Dilution Calculator

TC 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3628 mL | 16.8141 mL | 33.6281 mL | 67.2563 mL | 84.0704 mL |
| 5 mM | 0.6726 mL | 3.3628 mL | 6.7256 mL | 13.4513 mL | 16.8141 mL |
| 10 mM | 0.3363 mL | 1.6814 mL | 3.3628 mL | 6.7256 mL | 8.407 mL |
| 50 mM | 0.0673 mL | 0.3363 mL | 0.6726 mL | 1.3451 mL | 1.6814 mL |
| 100 mM | 0.0336 mL | 0.1681 mL | 0.3363 mL | 0.6726 mL | 0.8407 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyclo(D-Leu-L-Pro)
Catalog No.:BCN4028
CAS No.:36238-67-2
- Jatrorrhizine
Catalog No.:BCN5319
CAS No.:3621-38-3
- Columbamine
Catalog No.:BCN2722
CAS No.:3621-36-1
- NVP 231
Catalog No.:BCC4244
CAS No.:362003-83-6
- 2-Methoxyestradiol (2-MeOE2)
Catalog No.:BCC2228
CAS No.:362-07-2
- Genistein 7,4'-di-O-beta-D-glucopyranoside
Catalog No.:BCN7835
CAS No.:36190-98-4
- 3'-O-Methylorobol
Catalog No.:BCN5318
CAS No.:36190-95-1
- 3-Acetyl-2,5-dichlorothiophene
Catalog No.:BCC8602
CAS No.:36157-40-1
- Blumenol B
Catalog No.:BCN5317
CAS No.:36151-01-6
- Kobusin
Catalog No.:BCN7563
CAS No.:36150-23-9
- Dehydroleucodine
Catalog No.:BCN6897
CAS No.:36150-07-9
- Saxalin
Catalog No.:BCC8357
CAS No.:36150-06-8
- (±)-SLV 319
Catalog No.:BCC7954
CAS No.:362519-49-1
- Clemaphenol A
Catalog No.:BCN7834
CAS No.:362606-60-8
- Pitolisant
Catalog No.:BCC1862
CAS No.:362665-56-3
- Pitolisant oxalate
Catalog No.:BCC1864
CAS No.:362665-57-4
- Hederasaponin B
Catalog No.:BCN1085
CAS No.:36284-77-2
- Prostaglandin E2
Catalog No.:BCC7316
CAS No.:363-24-6
- Broussonol E
Catalog No.:BCN7996
CAS No.:363134-28-5
- Piroxicam
Catalog No.:BCC3841
CAS No.:36322-90-4
- YL-109
Catalog No.:BCC5543
CAS No.:36341-25-0
- Cyclo(L-Ala-L-Pro)
Catalog No.:BCN4012
CAS No.:36357-32-1
- Oxyphyllenone A
Catalog No.:BCN7103
CAS No.:363610-34-8
- 4-Methylhistamine dihydrochloride
Catalog No.:BCC7337
CAS No.:36376-47-3
Synthesis and Evaluation of Tricarbonyl (99m)Tc-Labeled 2-(4-Chloro)phenyl-imidazo[1,2-a]pyridine Analogs as Novel SPECT Imaging Radiotracer for TSPO-Rich Cancer.[Pubmed:27457557]
Int J Mol Sci. 2016 Jul 7;17(7). pii: ijms17071085.
The 18-kDa translocator protein (TSPO) levels are associated with brain, breast, and prostate cancer progression and have emerged as viable targets for cancer therapy and imaging. In order to develop highly selective and active ligands with a high affinity for TSPO, imidazopyridine-based TSPO ligand (CB256, 3) was prepared as the precursor. (99m)Tc- and Re-CB256 (1 and 2, respectively) were synthesized in high radiochemical yield (74.5% +/- 6.4%, decay-corrected, n = 5) and chemical yield (65.6%) by the incorporation of the [(99m)Tc(CO)(3)(H(2)O)(3)](+) and (NEt(4))(2)[Re(CO)(3)Br(3)] followed by HPLC separation. Radio-ligand 1 was shown to be stable (>99%) when incubated in human serum for 4 h at 37 degrees C with a relatively low lipophilicity (logD = 2.15 +/- 0.02). The rhenium-185 and -187 complex 2 exhibited a moderate affinity (Ki = 159.3 +/- 8.7 nM) for TSPO, whereas its cytotoxicity evaluated on TSPO-rich tumor cell lines was lower than that observed for the precursor. In vitro uptake studies of 1 in C6 and U87-MG cells for 60 min was found to be 9.84% +/- 0.17% and 7.87% +/- 0.23% ID, respectively. Our results indicated that (99m)Tc-CB256 can be considered as a potential new TSPO-rich cancer SPECT imaging agent and provides the foundation for further in vivo evaluation.
Coexistence of superconductivity and ferromagnetism in Sr0.5Ce0.5FBiS2-xSex (x = 0.5 and 1.0), a non-U material with Tc < TFM.[Pubmed:27892482]
Sci Rep. 2016 Nov 28;6:37527.
We have carried out detailed magnetic and transport studies of the new Sr0.5Ce0.5FBiS2-xSex (0.0 /= 0.5 in contrast to the undoped Sr0.5Ce0.5FBiS2 (x = 0) where magnetization measurements indicate a small superconducting volume fraction. Quite remarkably, as compared with the effective paramagnetic Ce-moment (~2.2 muB), the ferromagnetically ordered Ce-moment in the superconducting state is rather small (~0.1 muB) suggesting itinerant ferromagnetism. To the best of our knowledge, Sr0.5Ce0.5FBiS2-x Sex (x = 0.5 and 1.0) are distinctive Ce-based bulk superconducting itinerant ferromagnetic materials with Tc < TFM. Furthermore, a novel feature of these materials is that they exhibit a dual and quite unusual hysteresis loop corresponding to both the ferromagnetism and the coexisting bulk superconductivity.
Multiple endocrine neoplasia type 1 with anterior mediastinal parathyroid adenoma: successful localization using Tc-99m sestamibi SPECT/CT.[Pubmed:27904855]
Ann Surg Treat Res. 2016 Dec;91(6):323-326.
The most common manifestation of multiple endocrine neoplasia type 1 (MEN1) is hyperparathyroidism. Treatment of hyperparathyroidism in MEN patients is surgical removal of the parathyroid glands, however ectopic parathyroid gland is challenging for treatment. A 51-year-old female, the eldest of 3 MEN1 sisters, had hyperparathyroidism with ectopic parathyroid adenoma in the mediastinal para-aortic region, which was detected by technetium-99m (Tc-99m) sestamibi scintigraphy and single-photon emission computed tomography/computed tomography (SPECT/CT). She underwent total parathyroidectomy with video-assisted thoracoscopic surgery on an anterior mediastinal mass. Anterior mediastinal parathyroid adenoma in MEN1 patients is rare. Precise localization of an ectopic parathyroid gland with Tc-99m sestamibi SPECT/CT can lead to successful treatment of hyperparathyroidism. This is the first reported case in the literature of mediastinal parathyroid adenoma in MEN1 patient visualized by Tc-99m sestamibi SPECT/CT.
The predictive value of SS-16 in clinically diagnosed Parkinson's disease patients: comparison with (99m)Tc-TRODAT-1 SPECT scans.[Pubmed:27547386]
Transl Neurodegener. 2016 Aug 20;5:15.
BACKGROUND: Dopamine transporter based imaging has high diagnostic performance in distinguishing patients with Parkinson's disease (PD) from patients with non-Parkinsonian syndromes. Our previous study indicated that the "Sniffin' Sticks" odor identification test (SS-16) acts as a valid instrument for olfactory assessment in Chinese PD patients. The aim of the study was to compare the efficacy of the two methods in diagnosing PD. METHODS: Fifty-two PD patients were involved in this study and underwent single photon emission computed tomography (SPECT) imaging using the labeled dopamine transporter radiotracer (99m)Tc-TRODAT-1 to assess nigrostriatal dopaminergic function. Olfactory function was assessed with the "Sniffin' Sticks" odor identification test (SS-16) in all patients who received DAT-SPECT scanning. Statistical analysis (SPSS version 21) was carried out to determine the diagnostic accuracy of SS-16 as well as its correlation with (99m)Tc-TRODAT-1 SPECT, its positive predictive value (PPV), and negative predictive value (NPV). RESULTS: We identified a negative correlation between SS-16 and DAT SPECT (Kappa = 0.269, p = 0.004). By using the (99m)Tc-TRODAT-1 uptake results as the gold standard, the sensitivity and specificity of SS-16 was 56.8 and 37.5 %, respectively. Furthermore, the negative and positive predictive values were calculated as 13.6 and 83.3 %, respectively. CONCLUSIONS: SS-16 would not be used as a diagnostic tool for early stage PD patients. Negative results of SS-16 would not exclude the diagnosis of PD. Further tests are needed for validation.
Trishomocubanes: novel sigma ligands modulate cocaine-induced behavioural effects.[Pubmed:17113074]
Eur J Pharmacol. 2007 Jan 19;555(1):37-42.
Trishomocubane analogues TC1 (N-(3'-fluorophenyl)ethyl-4-azahexacyclo [5.4.1.0(2,6).0(3,10).0(5,9).0(8,11)]dodecan-3-ol) and TC4 (N-(3'-fluorophenyl)methyl-4-azahexacyclo [5.4.1.0(2,6).0(3,10).0(5,9).0(8,11)]dodecan-3-ol) were evaluated for their modulatory effects on locomotor activity as well as interactions with cocaine-induced responses. TC1 and TC4 have high affinity and moderate to high selectivity for sigma(1) (Ki=10 nM, sigma1/sigma2=0.03) and sigma2 (Ki=20 nM, sigma1/sigma2=7.6) receptor subtypes respectively. Both compounds have negligible affinity for the dopamine (DAT), serotonin (SERT), and norepinephrine (NET) transporters. In behavioural studies, TC1 produced a dose-related inhibition in spontaneous locomotor activity measured in a Digiscan apparatus. TC1 attenuated the stimulatory locomotor effect of 20 mg/kg cocaine with a half-maximal depressant activity (ID50) of 38.6 mg/kg. TC1 (dose range of 25 to 100 mg/kg) also partially substituted for the effect of cocaine (10 mg/kg) in a discriminative stimulus task, involving the trained discrimination between cocaine and saline using a two-lever choice method. Following a dose of 50 mg/kg TC1, a maximum of 31% substitution was reached. The response rate was reduced to 56% of vehicle control following a TC1 dose of 100 mg/kg. These behavioural effects suggest that TC1 can act as an antagonist via the sigma1 receptor. In contrast to TC1, TC4 produced a stimulant effect in locomotor activity with the ED50 estimated at 0.94 mg/kg. In addition, TC4 failed to inhibit cocaine-induced stimulation; neither did it substitute for the discriminative stimulus effects of cocaine. TC4 thus appears to interact predominantly with the sigma2 receptor subtype (sigma1/sigma2=7.6) which may result in dopamine stimulation independent of the effects of cocaine. The differential effect of TC1 and TC4 warrants further study of the mechanism of these actions. Present data also suggests a potential role for trishomocubane analogues in developing medication or research tools for cocaine addiction.


