4-Methylhistamine dihydrochlorideH4 receptor agonist CAS# 36376-47-3 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 36376-47-3 | SDF | Download SDF |
| PubChem ID | 215819 | Appearance | Powder |
| Formula | C6H13Cl2N3 | M.Wt | 198.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in water | ||
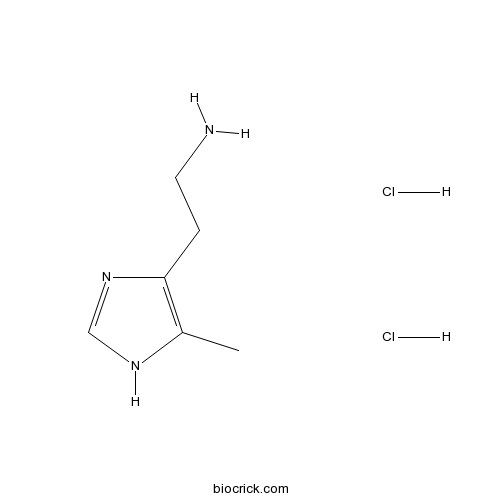
| Chemical Name | 2-(5-methyl-1H-imidazol-4-yl)ethanamine;dihydrochloride | ||
| SMILES | CC1=C(N=CN1)CCN.Cl.Cl | ||
| Standard InChIKey | BVJIXYJOFWYOBL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H11N3.2ClH/c1-5-6(2-3-7)9-4-8-5;;/h4H,2-3,7H2,1H3,(H,8,9);2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, high affinity H4 receptor agonist (Ki = 7 nM) that displays > 100-fold selectivity over other human histamine receptor subtypes. |

4-Methylhistamine dihydrochloride Dilution Calculator

4-Methylhistamine dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0482 mL | 25.2411 mL | 50.4821 mL | 100.9642 mL | 126.2053 mL |
| 5 mM | 1.0096 mL | 5.0482 mL | 10.0964 mL | 20.1928 mL | 25.2411 mL |
| 10 mM | 0.5048 mL | 2.5241 mL | 5.0482 mL | 10.0964 mL | 12.6205 mL |
| 50 mM | 0.101 mL | 0.5048 mL | 1.0096 mL | 2.0193 mL | 2.5241 mL |
| 100 mM | 0.0505 mL | 0.2524 mL | 0.5048 mL | 1.0096 mL | 1.2621 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
4-Methylhistamine dihydrochloride (or 4-methylhistamine) is a selective agonist of H4 receptor with a pEC50 value of 7.4±0.1 (α=1) [1].
In the chemotaxis of mast cells and leukocytes to sites of inflammation, the histamine H4 receptor (H4R) is involved [1].
In transfected cells, 4-methylhistamine bound to hH4R with the highest affinity, compared to the binding to other histamine receptors. The interaction between 4-methylhistamine and hH4R showed a higher selectivity than that between the drug and the H3R and H2R, and H1R by >100-fold and >100,000-fold, respectively. 4-Methylhistamine also had a high affinity for the rat and mouse H4R with Ki values of 73 and 55 nM, respectively, though this affinity was lower than that for hH4R. The agonistic effects of 4-methylhistamine to hH4R were antagonized by JNJ 7777120, a selective H4R antagonist. To the rat and mouse H4R, as a full H4 agonist, 4-methylhistamine showed pEC50 values of 5.6 ± 0.1 and 5.8 ± 0.1, respectively [1].
After being fasted for 18 h, rats were administered intraperitoneally (i.p.) with a single dose of 4-methylhistamine. 2 h later, they were subjected to a intra-articular (i.a.) injection of LPS. In both groups treated with 4-methylhistamine and LPS alone, respectively, the expression of TNF-α and NF-κB was increased, levels of IkB-α were decreased in synovial fluid and whole blood. Further, mRNA levels of IL-1β, TNF-α, and NF-κB were significantly increased. Western blot analysis results also confirmed that the expression of TNF-α, JAK-1, NF-κB and STAT-3 was increased in both 4-methylhistamine and LPS treatment groups. In the inflamed knee tissue of the JNJ 7777120-treated group, these increases were completely inhibited [2].
References:
[1]. Lim HD, van Rijn RM, Ling P, et al. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. Journal of Pharmacology and Experimental Therapeutics, 2005, 314(3): 1310-1321.
[2]. Ahmad SF, Ansari MA, Zoheir KMA, et al. Regulation of TNF-α and NF-κB activation through the JAK/STAT signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation. Immunobiology, 2015, 220(7): 889-898.
- Oxyphyllenone A
Catalog No.:BCN7103
CAS No.:363610-34-8
- Cyclo(L-Ala-L-Pro)
Catalog No.:BCN4012
CAS No.:36357-32-1
- YL-109
Catalog No.:BCC5543
CAS No.:36341-25-0
- Piroxicam
Catalog No.:BCC3841
CAS No.:36322-90-4
- Broussonol E
Catalog No.:BCN7996
CAS No.:363134-28-5
- Prostaglandin E2
Catalog No.:BCC7316
CAS No.:363-24-6
- Hederasaponin B
Catalog No.:BCN1085
CAS No.:36284-77-2
- Pitolisant oxalate
Catalog No.:BCC1864
CAS No.:362665-57-4
- Pitolisant
Catalog No.:BCC1862
CAS No.:362665-56-3
- Clemaphenol A
Catalog No.:BCN7834
CAS No.:362606-60-8
- (±)-SLV 319
Catalog No.:BCC7954
CAS No.:362519-49-1
- TC 1
Catalog No.:BCC7450
CAS No.:362512-81-0
- α,α'-Bis(4-hydroxy-3,5-dimethylphenyl)-1,4-diisopropylbenzene
Catalog No.:BCC9196
CAS No.:36395-57-0
- Metoclopramide
Catalog No.:BCC1743
CAS No.:364-62-5
- Diazoxide
Catalog No.:BCC6868
CAS No.:364-98-7
- A 419259
Catalog No.:BCC4307
CAS No.:364042-47-7
- N-p-trans-Coumaroyltyramine
Catalog No.:BCN5320
CAS No.:36417-86-4
- Tolperisone HCl
Catalog No.:BCC4740
CAS No.:3644-61-9
- 6-Hydroxystigmasta-4,22-dien-3-one
Catalog No.:BCN5321
CAS No.:36450-01-8
- 6beta-Hydroxystigmast-4-en-3-one
Catalog No.:BCN5322
CAS No.:36450-02-9
- Lucidenic acid SP1
Catalog No.:BCN7969
CAS No.:364622-33-3
- Doripenem Hydrate
Catalog No.:BCC1160
CAS No.:364622-82-2
- Ginsenoside Rk2
Catalog No.:BCN3721
CAS No.:364779-14-6
- Ginsenoside Rk3
Catalog No.:BCN3502
CAS No.:364779-15-7
Regulation of TNF-alpha and NF-kappaB activation through the JAK/STAT signaling pathway downstream of histamine 4 receptor in a rat model of LPS-induced joint inflammation.[Pubmed:25666529]
Immunobiology. 2015 Jul;220(7):889-98.
Histamine 4 receptor (H4R) is a novel target for the pharmacological modulation of histamine-mediated immune signals during inflammatory diseases. The purpose of this study was to assess the effects of the H4R agonist 4-Methylhistamine dihydrochloride (4-MeH) and antagonist JNJ7777120 (JNJ) in the inflamed rat knee. Animals were fasted for 18h before a single dose of 4-MeH or JNJ (30mg/kg) was administered intraperitoneally (i.p.), both followed by intra-articular (i.a.) injection of LPS 2h later. Blood and synovial fluid were collected after a short incubation period and TNF-alpha, NF-kappaB, and IkB-alpha levels were measured via flow cytometry. Additionally, we assessed the effects of H4R engagement on the expression of IL-1beta, TNF-alpha, and NF-kappaB mRNAs and the protein levels of TNF-alpha, NF-kappaB, JAK-1, and STAT-3 in the inflamed knee tissue. These results revealed increased TNF-alpha and NF-kappaB expression and decreased IkB-alpha levels in both the LPS alone and 4-MeH treated groups in whole blood and synovial fluid. Further, IL-1beta, TNF-alpha, and NF-kappaB mRNA levels were significantly increased and western blot analysis confirmed increased expression of TNF-alpha, NF-kappaB, JAK-1, and STAT-3 in both LPS and 4-MeH treatment groups. Furthermore, these increases were completely inhibited in the inflamed knee tissue of the JNJ-treated group. Thus, the inhibition of inflammatory mediators and signaling pathways by the H4R antagonist JNJ suggests the anti-arthritic importance of this molecule.
Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist.[Pubmed:15947036]
J Pharmacol Exp Ther. 2005 Sep;314(3):1310-21.
The histamine H(4) receptor (H(4)R) is involved in the chemotaxis of leukocytes and mast cells to sites of inflammation and is suggested to be a potential drug target for asthma and allergy. So far, selective H(4)R agonists have not been identified. In the present study, we therefore evaluated the human H(4)R (hH(4)R) for its interaction with various known histaminergic ligands. Almost all of the tested H(1)R and H(2)R antagonists, including several important therapeutics, displaced less than 30% of specific [(3)H]histamine binding to the hH(4)R at concentrations up to 10 microM. Most of the tested H(2)R agonists and imidazole-based H(3)R ligands show micromolar-to-nanomolar range hH(4)R affinity, and these ligands exert different intrinsic hH(4)R activities, ranging from full agonists to inverse agonists. Interestingly, we identified 4-methylhistamine as a high-affinity H(4)R ligand (K(i) = 50 nM) that has a >100-fold selectivity for the hH(4)R over the other histamine receptor subtypes. Moreover, 4-methylhistamine potently activated the hH(4)R (pEC(50) = 7.4 +/- 0.1; alpha = 1), and this response was competitively antagonized by the selective H(4)R antagonist JNJ 7777120 [1-[(5-chloro-1H-indol-2-yl)-carbonyl]-4-methylpiperazine] (pA(2) = 7.8). The identification of 4-methylhistamine as a potent H(4)R agonist is of major importance for future studies to unravel the physiological roles of the H(4)R.


