N-p-trans-CoumaroyltyramineCAS# 36417-86-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 36417-86-4 | SDF | Download SDF |
| PubChem ID | 5372945 | Appearance | White powder |
| Formula | C17H17NO3 | M.Wt | 283.3 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | Paprazine | ||
| Solubility | Soluble in methanol; slightly soluble in water | ||
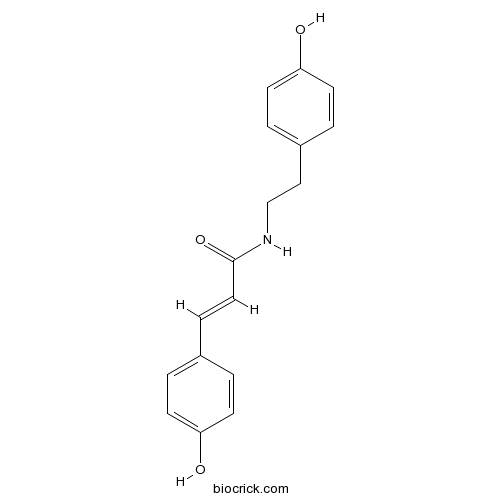
| Chemical Name | (E)-3-(4-hydroxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]prop-2-enamide | ||
| SMILES | C1=CC(=CC=C1CCNC(=O)C=CC2=CC=C(C=C2)O)O | ||
| Standard InChIKey | RXGUTQNKCXHALN-BJMVGYQFSA-N | ||
| Standard InChI | InChI=1S/C17H17NO3/c19-15-6-1-13(2-7-15)5-10-17(21)18-12-11-14-3-8-16(20)9-4-14/h1-10,19-20H,11-12H2,(H,18,21)/b10-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Paprazine may have cytotoxic activity. |
| In vitro | Chemical constituents from roots of Datura metel.[Pubmed: 29751713]Zhongguo Zhong Yao Za Zhi. 2018 Apr;43(8):1654-1661.To study the flavonoids of Scutellaria baicalensis and their bioactivities.
|
| Structure Identification | Nat Prod Res. 2009;23(15):1416-23.Alkaloids from Piper sarmentosum and Piper nigrum.[Pubmed: 19809914 ]Detailed chemical studies on the roots of Piper sarmentosum and Piper nigrum have resulted in several alkaloids. |

N-p-trans-Coumaroyltyramine Dilution Calculator

N-p-trans-Coumaroyltyramine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5298 mL | 17.6491 mL | 35.2983 mL | 70.5965 mL | 88.2457 mL |
| 5 mM | 0.706 mL | 3.5298 mL | 7.0597 mL | 14.1193 mL | 17.6491 mL |
| 10 mM | 0.353 mL | 1.7649 mL | 3.5298 mL | 7.0597 mL | 8.8246 mL |
| 50 mM | 0.0706 mL | 0.353 mL | 0.706 mL | 1.4119 mL | 1.7649 mL |
| 100 mM | 0.0353 mL | 0.1765 mL | 0.353 mL | 0.706 mL | 0.8825 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- A 419259
Catalog No.:BCC4307
CAS No.:364042-47-7
- Diazoxide
Catalog No.:BCC6868
CAS No.:364-98-7
- Metoclopramide
Catalog No.:BCC1743
CAS No.:364-62-5
- α,α'-Bis(4-hydroxy-3,5-dimethylphenyl)-1,4-diisopropylbenzene
Catalog No.:BCC9196
CAS No.:36395-57-0
- 4-Methylhistamine dihydrochloride
Catalog No.:BCC7337
CAS No.:36376-47-3
- Oxyphyllenone A
Catalog No.:BCN7103
CAS No.:363610-34-8
- Cyclo(L-Ala-L-Pro)
Catalog No.:BCN4012
CAS No.:36357-32-1
- YL-109
Catalog No.:BCC5543
CAS No.:36341-25-0
- Piroxicam
Catalog No.:BCC3841
CAS No.:36322-90-4
- Broussonol E
Catalog No.:BCN7996
CAS No.:363134-28-5
- Prostaglandin E2
Catalog No.:BCC7316
CAS No.:363-24-6
- Hederasaponin B
Catalog No.:BCN1085
CAS No.:36284-77-2
- Tolperisone HCl
Catalog No.:BCC4740
CAS No.:3644-61-9
- 6-Hydroxystigmasta-4,22-dien-3-one
Catalog No.:BCN5321
CAS No.:36450-01-8
- 6beta-Hydroxystigmast-4-en-3-one
Catalog No.:BCN5322
CAS No.:36450-02-9
- Lucidenic acid SP1
Catalog No.:BCN7969
CAS No.:364622-33-3
- Doripenem Hydrate
Catalog No.:BCC1160
CAS No.:364622-82-2
- Ginsenoside Rk2
Catalog No.:BCN3721
CAS No.:364779-14-6
- Ginsenoside Rk3
Catalog No.:BCN3502
CAS No.:364779-15-7
- Cinacalcet HCl
Catalog No.:BCC4408
CAS No.:364782-34-3
- Methylsynephrine Hydrochloride
Catalog No.:BCN3407
CAS No.:365-26-4
- Carnosic acid
Catalog No.:BCN5892
CAS No.:3650-09-7
- Agatholal
Catalog No.:BCN5323
CAS No.:3650-31-5
- beta-Costic acid
Catalog No.:BCN5324
CAS No.:3650-43-9
Cinnamoylphenethyl amides from Polygonum hyrcanicum possess anti-trypanosomal activity.[Pubmed:22816300]
Nat Prod Commun. 2012 Jun;7(6):753-5.
A methanolic extract from aerial parts of Polygonum hyrcanicum (Polygonaceae) showed high activity against Trypanosoma brucei rhodesiense (IC50 = 3.7 microg/mL). Bioassay-guided fractionation of the extract resulted in isolation of cinnamoylphenethyl amides, including N-trans-caffeoyltyramine (1), N-trans-p-coumaroyltyramine (7), and N-trans-feruloyltyramine (8) as the main active constituents (IC50s ranging from 2.2 to 13.3 microM). Some structurally related, but less active compounds, such as cannabisin B (2), tyrosol (3), p-coumaric acid (4), ferulic acid (5), and N-cis-feruloyltyramine (6) were also identified, along with N-trans-3,4-dimethoxycinnamoyldopamine (9). Cytotoxicity of the active compounds in L6 cells was determined, and selectivity indices (SI) of 7.9 to 33.4 were calculated.
Inhibitory effect of trans-N-p-coumaroyl tryamine from the twigs of Celtis chinensis on the acetylcholinesterase.[Pubmed:14560923]
Arch Pharm Res. 2003 Sep;26(9):735-8.
The methanolic extract of the twigs of Celtis chinensis was found to show inhibitory activity on acetylcholinesterase (AChE), an enzyme that plays a role in the metabolic hydrolysis of ACh. Bioassay-guided fractionation of the methanolic extract resulted in the isolation of N-p-coumaroyl tyramine, as an inhibitor on AChE. This compound inhibited AChE activity in a dose-dependent manner, and the IC50 value of trans-N-p-coumaroyl tyramine was 34.5 microg/mL (122 microM).
Interaction studies of coumaroyltyramine with human serum albumin and its biological importance.[Pubmed:20136105]
J Phys Chem B. 2010 Mar 4;114(8):3005-12.
N-trans-p-coumaroyltyramine (CT) isolated from Physalis minima is a phenolic substance exhibiting many pharmacological activities like potent inhibition of acetyl cholinesterase, cell proliferation, platelet aggregation, and also antioxidant activity. Here, we have studied the binding of CT with HSA at physiological pH 7.2 by using fluorescence, circular dichroism spectroscopy, mass spectrometry, and molecular docking methods. From the fluorescence emission studies, the number of binding sites and binding constant were calculated to be 2 and (4.5 +/- 0.01) x 10(5) M(-1), respectively. The free energy change was calculated as -7.6 kcal M(-1) at 25 degrees C, which indicates the hydrophobic interactions of CT with HSA and is in well agreement with the computational calculations and molecular docking studies. The changes in the secondary structure of HSA after its complexation with the ligand were studied with CD spectroscopy, which indicated that the protein became partially unfolded. Also, temperature did not affect the HSA-CT complexes. The binding of CT with HSA was detected as 2 molecules bound to HSA was determined using micro TOF-Q mass spectrometry. Further, molecular docking studies revealed that CT was binding at subdomain IIA with hydrophobic interactions and also by hydrogen-bond interactions between the hydroxyl (OH) group of carbon-16 and carbon-2 of CT and Arg222, Ala291, Val293, and Met298 of HSA, with hydrogen-bond distances of 2.488, 2.811, 2.678, and 2.586 A, respectively.
Amides from the stem of Capsicum annuum.[Pubmed:21425680]
Nat Prod Commun. 2011 Feb;6(2):227-9.
7'-(4'-hydroxyphenyl)-N-[(4-methoxyphenyl)ethyl]propenamide (1), 7'-(3',4'-dihydroxyphenyl)-N-[(4-methoxyphenyl)ethyl]propenamide (2), N-p-trans-Coumaroyltyramine (3), N-trans-caffeoyltyramine (4), beta-sitostenone (5), ferulic acid (6), hydroferulic acid (7), 5-hydroxy-3,4-dimethoxycinnamic acid (8), veratic acid (9), vanillic acid (10), isovanillic acid (11), syringic acid (12), (+)-syringaresinol (13), and pheophorbide a (14) were isolated from the stems of Capsicum annuum (Solanaceae). Among them, 1 is a new amide compound. The structures of these compounds were characterized and identified by spectral analyses.


