PiroxicamProstaglandin synthesis/Coxinhibitor CAS# 36322-90-4 |
- FK 3311
Catalog No.:BCC1576
CAS No.:116686-15-8
- Iguratimod
Catalog No.:BCC1641
CAS No.:123663-49-0
- Celecoxib
Catalog No.:BCC1099
CAS No.:169590-42-5
- Etoricoxib
Catalog No.:BCC1565
CAS No.:202409-33-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 36322-90-4 | SDF | Download SDF |
| PubChem ID | 54676228 | Appearance | Powder |
| Formula | C15H13N3O4S | M.Wt | 331.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (150.90 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
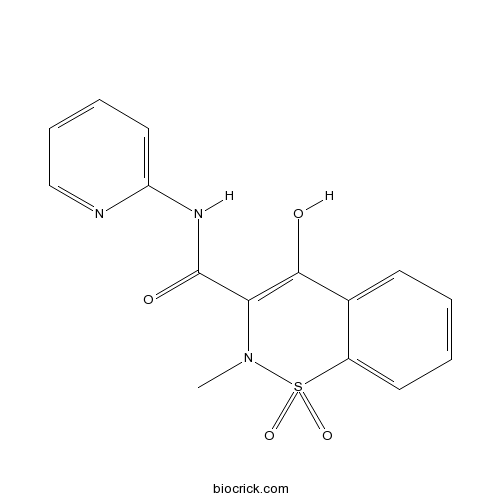
| Chemical Name | 4-hydroxy-2-methyl-1,1-dioxo-N-pyridin-2-yl-1$l^{6},2-benzothiazine-3-carboxamide | ||
| SMILES | CN1C(=C(C2=CC=CC=C2S1(=O)=O)O)C(=O)NC3=CC=CC=N3 | ||
| Standard InChIKey | QYSPLQLAKJAUJT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13N3O4S/c1-18-13(15(20)17-12-8-4-5-9-16-12)14(19)10-6-2-3-7-11(10)23(18,21)22/h2-9,19H,1H3,(H,16,17,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anti-inflammatory; highly selective inhibitor of COX-1 (ratio of IC50 values for COX-2/COX-1 ~ 600). |

Piroxicam Dilution Calculator

Piroxicam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.018 mL | 15.0898 mL | 30.1796 mL | 60.3591 mL | 75.4489 mL |
| 5 mM | 0.6036 mL | 3.018 mL | 6.0359 mL | 12.0718 mL | 15.0898 mL |
| 10 mM | 0.3018 mL | 1.509 mL | 3.018 mL | 6.0359 mL | 7.5449 mL |
| 50 mM | 0.0604 mL | 0.3018 mL | 0.6036 mL | 1.2072 mL | 1.509 mL |
| 100 mM | 0.0302 mL | 0.1509 mL | 0.3018 mL | 0.6036 mL | 0.7545 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Piroxicam is an effective and potent inhibitor of prostaglandin synthesis and a Cox-1 and Cox-2 inhibitor. Studies show that Piroxicam blocks release of platelet ADP, and inhibits Cox-1 (human prostaglandin H synthase isozyme-1, hPGHS-1) more potently than Cox-2 (hPGHS-2). Piroxicam is also a non-steroidal compound with anti-inflammatory properties.
- Broussonol E
Catalog No.:BCN7996
CAS No.:363134-28-5
- Prostaglandin E2
Catalog No.:BCC7316
CAS No.:363-24-6
- Hederasaponin B
Catalog No.:BCN1085
CAS No.:36284-77-2
- Pitolisant oxalate
Catalog No.:BCC1864
CAS No.:362665-57-4
- Pitolisant
Catalog No.:BCC1862
CAS No.:362665-56-3
- Clemaphenol A
Catalog No.:BCN7834
CAS No.:362606-60-8
- (±)-SLV 319
Catalog No.:BCC7954
CAS No.:362519-49-1
- TC 1
Catalog No.:BCC7450
CAS No.:362512-81-0
- Cyclo(D-Leu-L-Pro)
Catalog No.:BCN4028
CAS No.:36238-67-2
- Jatrorrhizine
Catalog No.:BCN5319
CAS No.:3621-38-3
- Columbamine
Catalog No.:BCN2722
CAS No.:3621-36-1
- NVP 231
Catalog No.:BCC4244
CAS No.:362003-83-6
- YL-109
Catalog No.:BCC5543
CAS No.:36341-25-0
- Cyclo(L-Ala-L-Pro)
Catalog No.:BCN4012
CAS No.:36357-32-1
- Oxyphyllenone A
Catalog No.:BCN7103
CAS No.:363610-34-8
- 4-Methylhistamine dihydrochloride
Catalog No.:BCC7337
CAS No.:36376-47-3
- α,α'-Bis(4-hydroxy-3,5-dimethylphenyl)-1,4-diisopropylbenzene
Catalog No.:BCC9196
CAS No.:36395-57-0
- Metoclopramide
Catalog No.:BCC1743
CAS No.:364-62-5
- Diazoxide
Catalog No.:BCC6868
CAS No.:364-98-7
- A 419259
Catalog No.:BCC4307
CAS No.:364042-47-7
- N-p-trans-Coumaroyltyramine
Catalog No.:BCN5320
CAS No.:36417-86-4
- Tolperisone HCl
Catalog No.:BCC4740
CAS No.:3644-61-9
- 6-Hydroxystigmasta-4,22-dien-3-one
Catalog No.:BCN5321
CAS No.:36450-01-8
- 6beta-Hydroxystigmast-4-en-3-one
Catalog No.:BCN5322
CAS No.:36450-02-9
Efficacy of a film-forming medical device containing sunscreen (50+) and piroxicam 0.8% in actinic keratosis and field cancerization: a multicenter, assessor-blinded, 3 month trial.[Pubmed:28358282]
Curr Med Res Opin. 2017 Jul;33(7):1255-1259.
INTRODUCTION: Sunscreen protection in subjects with actinic keratosis (AK) is highly recommended to prevent clinical evolution of this in situ skin cancer condition. Use of topical anti-cyclooxygenase drugs such as diclofenac and Piroxicam reduces the number of lesions and improves the cancerization field. A film-forming medical device in a cream formulation containing organic and inorganic sun-filters (50+ SPF) and Piroxicam 0.8% (ACTX) has shown in a pilot, single-center, open trial to reduce AK lesions improving the cancerization field. AIM: We evaluated in a multicenter, assessor-blinded, 3 month trial the efficacy of ACTX in AK. METHODS: A total of 70 subjects with at least three AK lesions on the scalp or face were enrolled after written informed consent. Primary outcomes of the study were the clinical evolution of number of AK lesions on a target zone area and the evolution of dermoscopy features of the target lesion, assessing erythema, scaling, pigmentation, and follicular plug, using a 5 point score (from 0 to 4; maximum score: 16). Lesion count and dermoscopy score were evaluated in a blind fashion assessing digital color high definition coded images. A secondary outcome was the Investigator Global Score (IGS) of clinical evolution of the target area using a 7 point scale from -2 (significantly worse) to +4 (completely cured). IGS was evaluated in an open fashion. Subjects were instructed to apply the cream twice daily on the target area, using one finger-tip unit for the treatment of a 35 cm(2) area. RESULTS: All but one subject (40 men and 30 women, mean age 73 years) concluded the study period. At baseline the mean (+/-SD) number of AK lesions in the target area were 7.0 (5.9) with a median value of 5 and the dermoscopy score of the target lesion was 7.0 (2.3) with a median value of 7.0. ACTX treatment reduced AK lesions to 3.2 (2.9), (p = .0001; Wilcoxon Test), representing a 55% relative reduction. Dermoscopy score was reduced to 3.3 (2.6) (p = .0001) (a reduction of 53%). The IGS after ACTX treatment was +1.9 (1.1), with a median of 2.0. A total of 86% of subjects showed a clinical improvement of IGS (>/=1) with a very significant/complete clearance (score +3 or +4) in 42% subjects. No change or a worsening of AK lesions was observed in 14% of the subjects. The product was well tolerated. No serious adverse events were reported during the duration of the trial. CONCLUSION: In this multicenter, assessor-blinded trial, the use of a film-forming medical device with sun protection and anti-inflammatory actions was effective in reducing AK lesions and improving the dermoscopy aspect of the target lesion in 86% of treated subjects. A head-to-head trial evaluating the efficacy of this medical device in comparison with diclofenac is warranted to establish whether this therapeutic approach could offer additional advantages in term of AK lesion reduction compared to an established topical treatment. (Trial ID: ISRCTN72020277).
Synergistic Effect of Carboplatin and Piroxicam on Two Bladder Cancer Cell Lines.[Pubmed:28373436]
Anticancer Res. 2017 Apr;37(4):1737-1745.
BACKGROUND/AIM: This study aimed to evaluate the in vitro efficacy of carboplatin and Piroxicam, both in isolation and combined, against T24 and 5637 human urinary bladder cancer cell lines. MATERIALS AND METHODS: Cell viability, drug interaction, cell morphology, cell proliferation, apoptosis and autophagy were analyzed after 72 h of drug exposure. Statistical analysis was performed and values of p<0.05 were considered statistically significant. RESULTS: Drug exposure in combination led to a significant reduction of cell viability comparatively to single-drug exposure. These combinations resulted in a synergistic interaction in the T24 (combination index for 50% effect (CI50)=0.65) and 5637 (CI50=0.17) cell lines. Notable increase of morphological alterations, a marked decrease of Ki-67 expression, a considerable enhancement of autophagic vacuoles and a minimal effect on apoptosis was observed in both cell lines treated with combined drugs. CONCLUSION: Data showed that in vitro combination of carboplatin and Piroxicam produced a more potent antiproliferative effect when compared to single drugs.
Effects of piroxicam on tissue distribution of sulfadimidine in West African Dwarf male and female goats.[Pubmed:28176534]
Hum Exp Toxicol. 2018 Jan;37(1):61-68.
Cases of Stevens-Johnson syndrome have been increasingly reported in Nigeria by individuals who consumed meat products of animals especially goats injected sulfonamides. Hence, tissue distribution and residues of intramuscular sulfadimidine were studied in West African Dwarf (WAD) goats. Twenty goats divided into two groups of 10 each (five males; five females) weighing 10.4 +/- 1.63 kg were administered intramuscular sulfadimidine (100 mg/kg body weight), and the second group was coadministered 5 mg/kg of Piroxicam via right and left thigh muscle, respectively. Samples of the liver, kidney, spleen, heart, lung, intestine, brain, and skeletal muscle were collected into sterile cellophane bags. Two untreated goats were killed and used for preparation of tissue standards. The tissue samples were stored frozen for analysis. High concentration of sulfadimidine residues was found in all the tissues of goats administered sulfadimidine as well as tissues of goats coadministered sulfadimidine/Piroxicam for up to 30 days postdrug administration. Generally, residues of sulfadimidine were observed to be significantly higher than the acceptable limit (0.1 ppm). Hence, consumption of meats from WAD goats administered sulfadimidine may pose very high risk of Stevens-Johnson syndrome in sensitive humans. As such consumption of such meats should be avoided.
Anti-Inflammatory Oxicams as Multi-donor Ligand Systems: pH- and Solvent-Dependent Coordination Modes of Meloxicam and Piroxicam to Ru and Os.[Pubmed:28198061]
Chemistry. 2017 Apr 6;23(20):4893-4902.
The nitrogen- and sulfur-containing 1,2-benzothiazines meloxicam and Piroxicam are widely used as nonsteroidal anti-inflammatory drugs. Intrigued by the presence of multiple donor atoms and therefore potentially rich coordination chemistry, we prepared a series of organometallic Ru and Os compounds with meloxicam and Piroxicam featuring either as mono- or bidentate ligand systems. The choice of the solvent and the pH value was identified as the critical parameter to achieve selectively mono- or bidentate coordination. The coordination modes were confirmed experimentally by NMR spectroscopy and single crystal X-ray diffraction analysis. Using DFT calculations, it was established that complexes in which meloxicam acts as a bidentate N,O donor are energetically more favorable than coordination as O,O and S,O donor systems. Since meloxicam and Piroxicam derivatives have shown anticancer activity in the past, we aimed to compare the complexes with mono- and bidentate ligands on their in vitro anticancer activity. However, stability studies revealed that only the latter complexes were stable in [D6 ]DMSO/D2 O (5:95) and therefore no direct comparisons could be made. The meloxicam complexes 1 and 2 showed moderate cytotoxicity, whereas the Piroxicam derivatives 5 and 6 were hardly active against the utilized cell lines.


