PitolisantNonimidazole inverse agonist CAS# 362665-56-3 |
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 362665-56-3 | SDF | Download SDF |
| PubChem ID | 9948102 | Appearance | Powder |
| Formula | C17H26ClNO | M.Wt | 295.85 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
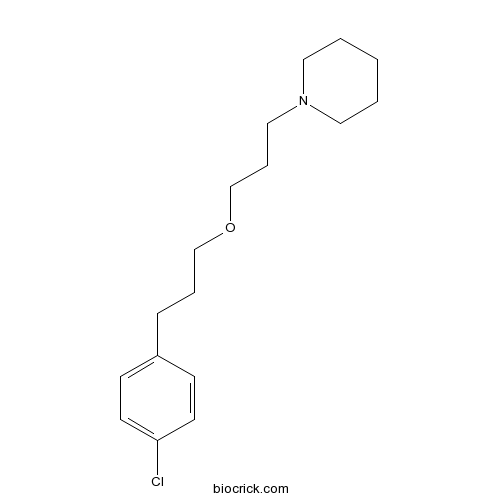
| Chemical Name | 1-[3-[3-(4-chlorophenyl)propoxy]propyl]piperidine | ||
| SMILES | C1CCN(CC1)CCCOCCCC2=CC=C(C=C2)Cl | ||
| Standard InChIKey | NNACHAUCXXVJSP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pitolisant Dilution Calculator

Pitolisant Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3801 mL | 16.9005 mL | 33.8009 mL | 67.6018 mL | 84.5023 mL |
| 5 mM | 0.676 mL | 3.3801 mL | 6.7602 mL | 13.5204 mL | 16.9005 mL |
| 10 mM | 0.338 mL | 1.69 mL | 3.3801 mL | 6.7602 mL | 8.4502 mL |
| 50 mM | 0.0676 mL | 0.338 mL | 0.676 mL | 1.352 mL | 1.69 mL |
| 100 mM | 0.0338 mL | 0.169 mL | 0.338 mL | 0.676 mL | 0.845 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 0.16 nM(Ki value); 1.5 nM(EC50) [1] Pitolisant (BF2.649) is a novel, potent, and selective nonimidazole inverse agonist at the recombinant human H3 receptor. BF2.649 behaved as a competitive antagonist with a Ki value of 0.16 nM and as an inverse agonist with an EC50 value of 1.5 nM and an intrinsic activity approximately 50% higher than that of ciproxifan. in vitro: BF2.649 behaved as a competitive antagonist with a Ki value of 0.16 nM and as an inverse agonist with an EC50 value of 1.5 nM and an intrinsic activity approximately 50% higher than that of ciproxifan. Pitolisant in vitro potency was approximately 6 times lower at the rodent receptor [1]. in vivo: In mice, the oral bioavailability coefficient, i.e., the ratio of plasma areas under the curve after oral and i.v. administrations, respectively, was 84%. BF2.649 dose dependently enhanced tele-methylhistamine levels in mouse brain, an index of histaminergic neuron activity, with an ED50 value of 1.6 mg/kg p.o., a response that persisted after repeated administrations for 17 days [1]. A statistically significant suppressive effect (standardized photosensitive response [SPR] reduction as measured with paired t-tests) for 20-, 40-, or 60-mg doses of pitolisant was seen in 9/14 (64%) patients of whom 6/14 (43%) showed abolition of the response to intermittent photic stimulation (IPS) [2]. BF2.649 showed significant inhibitory activity in several mouse models of schizophrenia [3]. Clinical trial: HARMONYIII: Long-term, Open-label Study in Narcolepsy With BF2.649 (Pitolisant). Phase3
- Clemaphenol A
Catalog No.:BCN7834
CAS No.:362606-60-8
- (±)-SLV 319
Catalog No.:BCC7954
CAS No.:362519-49-1
- TC 1
Catalog No.:BCC7450
CAS No.:362512-81-0
- Cyclo(D-Leu-L-Pro)
Catalog No.:BCN4028
CAS No.:36238-67-2
- Jatrorrhizine
Catalog No.:BCN5319
CAS No.:3621-38-3
- Columbamine
Catalog No.:BCN2722
CAS No.:3621-36-1
- NVP 231
Catalog No.:BCC4244
CAS No.:362003-83-6
- 2-Methoxyestradiol (2-MeOE2)
Catalog No.:BCC2228
CAS No.:362-07-2
- Genistein 7,4'-di-O-beta-D-glucopyranoside
Catalog No.:BCN7835
CAS No.:36190-98-4
- 3'-O-Methylorobol
Catalog No.:BCN5318
CAS No.:36190-95-1
- 3-Acetyl-2,5-dichlorothiophene
Catalog No.:BCC8602
CAS No.:36157-40-1
- Blumenol B
Catalog No.:BCN5317
CAS No.:36151-01-6
- Pitolisant oxalate
Catalog No.:BCC1864
CAS No.:362665-57-4
- Hederasaponin B
Catalog No.:BCN1085
CAS No.:36284-77-2
- Prostaglandin E2
Catalog No.:BCC7316
CAS No.:363-24-6
- Broussonol E
Catalog No.:BCN7996
CAS No.:363134-28-5
- Piroxicam
Catalog No.:BCC3841
CAS No.:36322-90-4
- YL-109
Catalog No.:BCC5543
CAS No.:36341-25-0
- Cyclo(L-Ala-L-Pro)
Catalog No.:BCN4012
CAS No.:36357-32-1
- Oxyphyllenone A
Catalog No.:BCN7103
CAS No.:363610-34-8
- 4-Methylhistamine dihydrochloride
Catalog No.:BCC7337
CAS No.:36376-47-3
- α,α'-Bis(4-hydroxy-3,5-dimethylphenyl)-1,4-diisopropylbenzene
Catalog No.:BCC9196
CAS No.:36395-57-0
- Metoclopramide
Catalog No.:BCC1743
CAS No.:364-62-5
- Diazoxide
Catalog No.:BCC6868
CAS No.:364-98-7
Action of Pitolisant on the stimulant and rewarding effects of cocaine in mice.[Pubmed:27568835]
Eur J Pharmacol. 2016 Nov 15;791:552-559.
Previous studies have demonstrated that the histamine H3 receptor inverse agonist thioperamide potentiates the stimulant and rewarding effects of cocaine. However, these potentiating effects of thioperamide do not necessarily result from H3 receptor blockade since thioperamide is an imidazole-based compound capable of enhancing plasma cocaine concentrations by blocking cytochrome P450 activity. In contrast, Pitolisant is a non-imidazole H3 receptor inverse agonist that has already been tested in clinical trials but it remains to be determined whether this compound also potentiates the behavioral effects of cocaine. The present study tested the effects of Pitolisant on locomotion, on cocaine-induced hyperactivity and on the development of conditioned place preference induced by cocaine (2 and 8mg/kg, i.p.) in male C57BL/6J mice. Pitolisant was injected 30min before each cocaine-pairing session. Locomotion recorded on the first cocaine-pairing session was used to test the effects of Pitolisant on the locomotor effects of cocaine. Our results show that doses of Pitolisant higher than 10mg/kg depressed locomotion. When injected alone at doses that did not affect locomotion, Pitolisant (2.5-10mg/kg, i.p.) had no rewarding properties in the place conditioning technique. Additionally, Pitolisant did not significantly alter cocaine-induced hyperactivity and cocaine-induced conditioned place preference. Taken together, our study indicates that Pitolisant has no addictive properties alone. Moreover, this compound does not significantly affect the stimulant and rewarding effects of cocaine. These results add further evidence to support the hypothesis that a pharmacokinetic interaction is involved in the ability of thioperamide to potentiate cocaine's psychomotor effects.
Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial.[Pubmed:28129985]
Lancet Neurol. 2017 Mar;16(3):200-207.
BACKGROUND: Histaminergic neurons are crucial to maintain wakefulness, but their role in cataplexy is unknown. We assessed the safety and efficacy of Pitolisant, a histamine H3 receptor inverse agonist, for treatment of cataplexy in patients with narcolepsy. METHODS: For this randomised, double-blind, placebo-controlled trial we recruited patients with narcolepsy from 16 sleep centres in nine countries (Bulgaria, Czech Republic, Hungary, Macedonia, Poland, Russia, Serbia, Turkey, and Ukraine). Patients were eligible if they were aged 18 years or older, diagnosed with narcolepsy with cataplexy according to version two of the International Classification of Sleep Disorders criteria, experienced at least three cataplexies per week, and had excessive daytime sleepiness (defined as an Epworth Sleepiness Scale score >/=12). We used a computer-generated sequence via an interactive web response system to randomly assign patients to receive either Pitolisant or placebo once per day (1:1 ratio). Randomisation was done in blocks of four. Participants and investigators were masked to treatment allocation. Treatment lasted for 7 weeks: 3 weeks of flexible dosing decided by investigators according to efficacy and tolerance (5 mg, 10 mg, or 20 mg oral Pitolisant), followed by 4 weeks of stable dosing (5 mg, 10 mg, 20 mg, or 40 mg). The primary endpoint was the change in the average number of cataplexy attacks per week as recorded in patient diaries (weekly cataplexy rate [WCR]) between the 2 weeks of baseline and the 4 weeks of stable dosing period. Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT01800045. FINDINGS: The trial was done between April 19, 2013, and Jan 28, 2015. We screened 117 patients, 106 of whom were randomly assigned to treatment (54 to Pitolisant and 52 to placebo) and, after dropout, 54 patients from the Pitolisant group and 51 from the placebo group were included in the intention-to-treat analysis. The WCR during the stable dosing period compared with baseline was decreased by 75% (WCRfinal=2.27; WCRbaseline=9.15; WCRfinal/baseline=0.25) in patients who received Pitolisant and 38% (WCRfinal=4.52; WCRbaseline=7.31; WCRfinal/baseline=0.62) in patients who received placebo (rate ratio 0.512; 95% CI 0.43-0.60, p<0.0001). Treatment-related adverse events were significantly more common in the Pitolisant group than in the placebo group (15 [28%] of 54 vs 6 [12%] of 51; p=0.048). There were no serious adverse events, but one case of severe nausea in the Pitolisant group. The most frequent adverse events in the Pitolisant group (headache, irritability, anxiety, and nausea) were mild or moderate except one case of severe nausea. No withdrawal syndrome was detected following Pitolisant treatment; one case was detected in the placebo group. INTERPRETATION: Pitolisant was well tolerated and efficacious in reducing cataplexy. If confirmed in long-term studies, Pitolisant might constitute a useful first-line therapy for cataplexy in patients with narcolepsy, for whom there are currently few therapeutic options. FUNDING: Bioprojet, France.
Pitolisant for narcolepsy.[Pubmed:28087755]
Drug Ther Bull. 2017 Jan;55(1):6-8.
Pitolisant (Wakix-Bioprojet Pharma) is a new treatment for adults with narcolepsy with or without cataplexy. It was licensed for use in the EU in March last year and has orphan drug status.(1) Here, we consider the evidence for Pitolisant for the treatment of narcolepsy in adults and how it fits with current management strategies.


