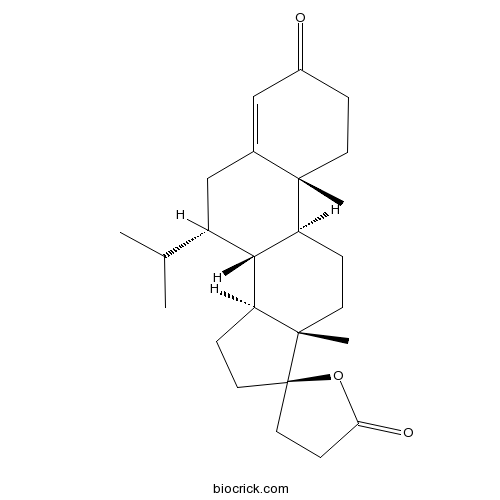
RU 26752Mineralocorticoid receptor antagonist CAS# 76676-33-0 |
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- INCB8761(PF-4136309)
Catalog No.:BCC1649
CAS No.:1341224-83-6
- MK-0812
Catalog No.:BCC1755
CAS No.:624733-88-6
- INCB 3284 dimesylate
Catalog No.:BCC1646
CAS No.:887401-93-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 76676-33-0 | SDF | Download SDF |
| PubChem ID | 122293 | Appearance | Powder |
| Formula | C25H36O3 | M.Wt | 384.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in DMSO | ||
| Chemical Name | (7S,8R,9S,10R,13S,14S,17R)-10,13-dimethyl-7-propan-2-ylspiro[2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthrene-17,5'-oxolane]-2',3-dione | ||
| SMILES | CC(C)C1CC2=CC(=O)CCC2(C3C1C4CCC5(C4(CC3)C)CCC(=O)O5)C | ||
| Standard InChIKey | GIMMPTDKHYFNQT-BTRFTSHOSA-N | ||
| Standard InChI | InChI=1S/C25H36O3/c1-15(2)18-14-16-13-17(26)5-9-23(16,3)19-6-10-24(4)20(22(18)19)7-11-25(24)12-8-21(27)28-25/h13,15,18-20,22H,5-12,14H2,1-4H3/t18-,19-,20-,22+,23-,24-,25+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mineralocorticoid receptor antagonist. Prevents aldosterone-induced hypertension in rats. |

RU 26752 Dilution Calculator

RU 26752 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6004 mL | 13.0022 mL | 26.0044 mL | 52.0088 mL | 65.0111 mL |
| 5 mM | 0.5201 mL | 2.6004 mL | 5.2009 mL | 10.4018 mL | 13.0022 mL |
| 10 mM | 0.26 mL | 1.3002 mL | 2.6004 mL | 5.2009 mL | 6.5011 mL |
| 50 mM | 0.052 mL | 0.26 mL | 0.5201 mL | 1.0402 mL | 1.3002 mL |
| 100 mM | 0.026 mL | 0.13 mL | 0.26 mL | 0.5201 mL | 0.6501 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Detomidine
Catalog No.:BCC4079
CAS No.:76631-46-4
- BAM 22P
Catalog No.:BCC5797
CAS No.:76622-26-9
- beta-D-Fructopyranose
Catalog No.:BCC8176
CAS No.:7660-25-5
- Imidazo[1,2-b]pyridazine
Catalog No.:BCC9001
CAS No.:766-55-2
- 3-Ethyl-4-methyl-3-pyrrolin-2-one
Catalog No.:BCC8632
CAS No.:766-36-9
- OR-486
Catalog No.:BCC5661
CAS No.:7659-29-2
- Divalproex Sodium
Catalog No.:BCC4379
CAS No.:76584-70-8
- SR 202
Catalog No.:BCC7243
CAS No.:76541-72-5
- Heraclenol 3'-O-[beta-D-apiofuranosyl-(1-6)-beta-D-glucopyranoside]
Catalog No.:BCN1362
CAS No.:765316-44-7
- 1-2-Cyclohexanedione
Catalog No.:BCN2265
CAS No.:765-87-7
- 10-Hydroxy-2-decenoic acid
Catalog No.:BCN2654
CAS No.:765-01-5
- 15,16-Dinor-8(17),11-labdadien-13-one
Catalog No.:BCN4312
CAS No.:76497-69-3
- RU 28318, potassium salt
Catalog No.:BCC7146
CAS No.:76676-34-1
- E-64-c
Catalog No.:BCC3588
CAS No.:76684-89-4
- 1-Deacetylnimbolinin B
Catalog No.:BCN4313
CAS No.:76689-98-0
- Mallorepine
Catalog No.:BCN4317
CAS No.:767-98-6
- Decumbenine
Catalog No.:BCC8312
CAS No.:76733-83-0
- Pyronaridine Tetraphosphate
Catalog No.:BCC1144
CAS No.:76748-86-2
- Lupalbigenin
Catalog No.:BCN4314
CAS No.:76754-24-0
- GBR 12935
Catalog No.:BCC5381
CAS No.:76778-22-8
- 2'-Hydroxydaidzein
Catalog No.:BCN4585
CAS No.:7678-85-5
- 3-Hydroxy-4',5,7-trimethoxyflavanone
Catalog No.:BCN4316
CAS No.:76792-94-4
- Azathramycin
Catalog No.:BCC1392
CAS No.:76801-85-9
- Potassium Iodide
Catalog No.:BCC4826
CAS No.:7681-11-0
Corticosterone is a preferable ligand for measuring rat brain corticosteroid receptors: competition by RU 28362 and RU 26752 for dexamethasone binding in rat hippocampal cytosol.[Pubmed:8242402]
Brain Res. 1993 Oct 15;625(1):84-92.
It is unclear whether in vitro corticosteroid receptor binding assays have used inappropriately high concentrations of synthetic corticosteroid competitors, thereby potentially introducing error into estimates of type I (mineralocorticoid) and type II (glucocorticoid) receptor binding. To determine more accurately the concentration of blockers necessary to discriminate between these two sites, we have derived Ki values for the competition of dexamethasone, RU 28362 and RU 26752 for [3H]corticosterone and [3H]dexamethasone binding in rat hippocampus. Non-specific binding of both radioligands was defined with unlabeled dexamethasone to exclude transcortin. The type II agonist RU 28362 competed for only a portion of [3H]corticosterone binding, exhibiting a Ki of 0.5 nM for this binding. In contrast, RU 28362 fully competed all binding of a saturating concentration of [3H]dexamethasone, even though [3H]dexamethasone also recognized type I receptors, defined as specific [3H]corticosterone binding in the presence of 80 nM RU 28362. RU 28362 competition for [3H]dexamethasone binding exhibited characteristics of a 2-site interaction, with Kis of 0.3 and 194 nM. The type I receptor antagonist RU 26752 competed less effectively for [3H]corticosterone and [3H]dexamethasone binding, but nonetheless competed fully within a 1000-fold concentration range. Even at a level less than 125 x its Ki for type I binding, RU 26752 still inhibited virtually all type II receptor binding by [3H]corticosterone. We conclude that type I and II receptors in rat brain are best distinguished using [3H]corticosterone as the labelling ligand, with cold RU 28362 and dexamethasone to eliminate binding to type II and transcortin sites, respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Effects of antimineralocorticoid RU 26752 on steroid-induced hypertension in rats.[Pubmed:2333987]
Am J Physiol. 1990 May;258(5 Pt 1):E737-9.
The effect of mineralocorticoid antagonist RU 26752 on the development and maintenance of hypertension produced by long-term administration of mineralocorticoid agonist aldosterone has been investigated. Uninephrectomized, saline-drinking male Sprague-Dawley rats were subcutaneously implanted with either placebo (control) pellets or pellets containing 100 micrograms aldosterone, 50 mg RU 26752, or 100 micrograms aldosterone plus 50 mg RU 26752. Aldosterone treatment resulted in an increase in blood pressure to 165 +/- 5 mmHg over the control value of 105 +/- 2 mmHg within 3 wk of experimental period. RU 26752 given alone had no observable hypertensinogenic effect. However, RU 26752 administered with aldosterone significantly prevented the hypertension produced by aldosterone alone. RU 26752 when given with aldosterone was able to prevent the aldosterone-induced increase in saline consumption, increase urine output, and reduce urinary Na+ excretion. The results presented suggest that long-term administration of antimineralocorticoid RU 26752 in vivo to Sprague-Dawley rats prevents the aldosterone-induced hypertension.
Structural basis of spirolactone recognition by the mineralocorticoid receptor.[Pubmed:17569793]
Mol Pharmacol. 2007 Sep;72(3):563-71.
Spirolactones are potent antagonists of the mineralocorticoid receptor (MR), a ligand-induced transcription factor belonging to the nuclear receptor superfamily. Spirolactones are synthetic molecules characterized by the presence of a C17 gamma-lactone, which is responsible for their antagonist character. They harbor various substituents at several positions of the steroid skeleton that modulate their potency in ways that remain to be determined. This is particularly obvious for C7 substituents. The instability of antagonist-MR complexes makes them difficult to crystallize. We took advantage of the S810L activating mutation in MR (MR(S810L)), which increases the stability of ligand-MR complexes to crystallize the ligand-binding domain (LBD) of MR(S810L) associated with 7alpha-acetylthio-17beta-hydroxy-3-oxopregn-4-en-21-carboxylic acid gamma-lactone (SC9420), a spirolactone with a C7 thioacetyl group. The crystal structure makes it possible to identify the contacts between SC9420 and MR and to elucidate the role of Met852 in the mode of accommodation of the C7 substituent of SC9420. The transactivation activities of MR(S810L/Q776A), MR(S810L/R817A), and MR(S810L/N770A) reveal that the contacts between SC9420 and the Gln776 and Arg817 residues are crucial to maintaining MR(S810L) in its active state, whereas the contact between SC9420 and the Asn770 residue contributes only to the high affinity of SC9420 for MR. Moreover, docking experiments with other C7-substituted spirolactones revealed that the MR(S810L)-activating potency of spirolactones is linked to the ability of their C7 substituent to be accommodated in LBD. It is remarkable that the MR(S810L)-activating and MR(WT)-inactivating potencies of the C7-substituted spirolactones follow the same order, suggesting that the C7 substituent is accommodated in the same way in MR(S810L) and MR(WT). Thus, the MR(S810L) structure may provide a powerful tool for designing new, more effective, MR antagonists.


