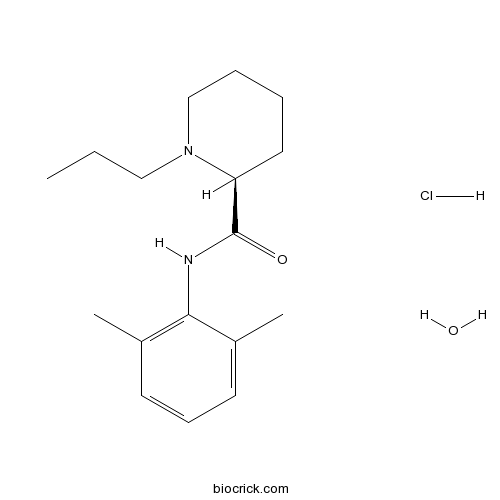
Ropivacaine hydrochloride monohydrateanaesthetic, long-acting and local CAS# 132112-35-7 |
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 132112-35-7 | SDF | Download SDF |
| PubChem ID | 6918111 | Appearance | Powder |
| Formula | C17H29ClN2O2 | M.Wt | 328.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 14 mg/mL (42.57 mM; Need ultrasonic and warming) | ||
| Chemical Name | (2S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide;hydrate;hydrochloride | ||
| SMILES | O.[Cl-].CCCN1CCCC[C@H]1C(=O)Nc2c(C)cccc2C.[H+] | ||
| Standard InChIKey | VSHFRHVKMYGBJL-CKUXDGONSA-N | ||
| Standard InChI | InChI=1S/C17H26N2O.ClH.H2O/c1-4-11-19-12-6-5-10-15(19)17(20)18-16-13(2)8-7-9-14(16)3;;/h7-9,15H,4-6,10-12H2,1-3H3,(H,18,20);1H;1H2/t15-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ropivacaine hydrochloride monohydrate Dilution Calculator

Ropivacaine hydrochloride monohydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0406 mL | 15.2031 mL | 30.4062 mL | 60.8125 mL | 76.0156 mL |
| 5 mM | 0.6081 mL | 3.0406 mL | 6.0812 mL | 12.1625 mL | 15.2031 mL |
| 10 mM | 0.3041 mL | 1.5203 mL | 3.0406 mL | 6.0812 mL | 7.6016 mL |
| 50 mM | 0.0608 mL | 0.3041 mL | 0.6081 mL | 1.2162 mL | 1.5203 mL |
| 100 mM | 0.0304 mL | 0.152 mL | 0.3041 mL | 0.6081 mL | 0.7602 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ropivacaine hydrochloride monohydrate is an effective, long-acting and local anaesthetic [1].
In COS-7 cells, Ropivacaine hydrochloride monohydrate has been reported to inhibit the the SUR2A/Kir6.2 channel currents. The inhibitory effects of Ropivacaine have shown be concentration-dependent and reversible because the channel currents recovered after washout. Furthermore, the IC50 values of Ropivacaine hydrochloride monohydrate are 249±11, 2235±115, 2442±132 and 2375±179μM for SUR2A/Kir6.2, SUR2B/Kir6.1, SUR2B/Kir6.2 and Kir6.2△C36, respectively. Apart from these, Ropivacaine hydrochloride monohydrate has also demonstrated to inhibit sarcolemmal KATP channels in the cardiovascular system by a stereoselective and tissue-specific way[2].
References:
[1] Reiz S1, Häggmark S, Johansson G, Nath S. Cardiotoxicity of ropivacaine--a new amide local anaesthetic agent. Acta Anaesthesiol Scand. 1989 Feb;33(2):93-8.
[2] Kawano T1, Oshita S, Takahashi A, Tsutsumi Y, Tomiyama Y, Kitahata H, Kuroda Y, Nakaya Y. Molecular mechanisms of the inhibitory effects of bupivacaine, levobupivacaine, and ropivacaine on sarcolemmal adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Anesthesiology. 2004 Aug;101(2):390-8.
- Marmesin angelate
Catalog No.:BCN8139
CAS No.:13209-79-5
- Cryptoacetalide
Catalog No.:BCN3139
CAS No.:132059-23-5
- UNC 0631
Catalog No.:BCC4143
CAS No.:1320288-19-4
- UNC 0646
Catalog No.:BCC2431
CAS No.:1320288-17-2
- Dihydrocucurbitacin B
Catalog No.:BCN3118
CAS No.:13201-14-4
- Benzydamine HCl
Catalog No.:BCC4637
CAS No.:132-69-4
- Pheniramine Maleate
Catalog No.:BCC4700
CAS No.:132-20-7
- Diphenylpyraline HCl
Catalog No.:BCC3768
CAS No.:132-18-3
- Benztropine mesylate
Catalog No.:BCC4524
CAS No.:132-17-2
- Shizukaol A
Catalog No.:BCN6984
CAS No.:131984-98-0
- 3,6,19-Trihydroxy-23-oxo-12-ursen-28-oic acid
Catalog No.:BCN1584
CAS No.:131984-82-2
- CC0651
Catalog No.:BCC4200
CAS No.:1319207-44-7
- O,O-diacetyldaurisoline
Catalog No.:BCC8221
CAS No.:132139-17-4
- Epi-Cryptoacetalide
Catalog No.:BCN3140
CAS No.:132152-57-9
- Dracoflavan A
Catalog No.:BCN3588
CAS No.:132185-42-3
- 5alpha-Hydroxycostic acid
Catalog No.:BCN6169
CAS No.:132185-83-2
- 5beta-Hydroxycostic acid
Catalog No.:BCN6170
CAS No.:132185-84-3
- (2R,3S)-3-Phenylisoserine hydrochloride
Catalog No.:BCN8527
CAS No.:132201-32-2
- N-Benzoyl-(2R,3S)-3-phenylisoserine
Catalog No.:BCN8525
CAS No.:132201-33-3
- Cilnidipine
Catalog No.:BCC1083
CAS No.:132203-70-4
- 3,6-Dibenzyl-2-hydroxy-5-methoxypyrazine
Catalog No.:BCN7335
CAS No.:132213-65-1
- Alyxialactone
Catalog No.:BCN6171
CAS No.:132237-63-9
- Rubioncolin C
Catalog No.:BCN7871
CAS No.:132242-52-5
- Salpriolactone
Catalog No.:BCN3220
CAS No.:132278-72-9
Lack of metabolic racemisation of ropivacaine, determined by liquid chromatography using a chiral AGP column.[Pubmed:7640170]
Chirality. 1995;7(4):272-7.
Ropivacaine hydrochloride monohydrate (ropivacaine) is a new local anaesthetic agent which is administered exclusively as the (-)-(S)-form. The aim of the study was to determine whether metabolic racemisation of (-)-(S)-ropivacaine occurs. This was tested in man, rat, dog, and sheep after different routes of administration. The enantiomers of ropivacaine and two of the major metabolites, 3-hydroxy-ropivacaine and 2',6'-pipecoloxylidide (PPX), were determined in urine samples by liquid chromatography on a Chiral AGP column after liquid-liquid extraction. It was possible to detect < 1% of the (+)-(R)-enantiomer of both ropivacaine and the two major metabolites. In the samples examined, no trace of metabolic racemisation was observed. In pharmacokinetic, pharmacodynamic, toxicological, and metabolic studies, therefore, nonchiral assays are considered to be adequate.


