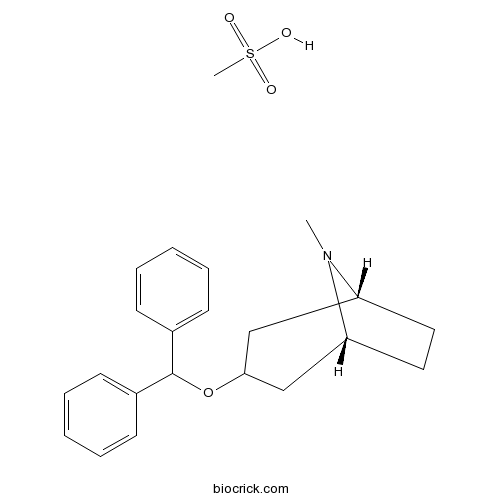
Benztropine mesylateCAS# 132-17-2 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 132-17-2 | SDF | Download SDF |
| PubChem ID | 8584 | Appearance | Powder |
| Formula | C22H29NO4S | M.Wt | 403.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Benzatropine mesylate; Benzotropine mesylate; Benztropine methanesulfonate | ||
| Solubility | H2O : ≥ 200 mg/mL (495.61 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (1R,5R)-3-benzhydryloxy-8-methyl-8-azabicyclo[3.2.1]octane;methanesulfonic acid | ||
| SMILES | CN1C2CCC1CC(C2)OC(C3=CC=CC=C3)C4=CC=CC=C4.CS(=O)(=O)O | ||
| Standard InChIKey | CPFJLLXFNPCTDW-STYNFMPRSA-N | ||
| Standard InChI | InChI=1S/C21H25NO.CH4O3S/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17;1-5(2,3)4/h2-11,18-21H,12-15H2,1H3;1H3,(H,2,3,4)/t18-,19-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Benzotropine is a centrally-acting, antimuscarinic agent used as an adjunct in the treatment of Parkinson's disease.
Target: mAChR
Benzotropine is a centrally-acting, antimuscarinic agent used as an adjunct in the treatment of Parkinson's disease. It may also be used to treat extrapyramidal reactions, such as dystonia and Parkinsonism, caused by antipsychotics. Symptoms of Parkinson's disease and extrapyramidal reactions arise from decreases in dopaminergic activity which creates an imbalance between dopaminergic and cholinergic activity. Anticholinergic therapy is thought to aid in restoring this balance leading to relief of symptoms. In addition to its anticholinergic effects, benztropine also inhibits the reuptake of dopamine at nerve terminals via the dopamine transporter. Benzotropine also produces antagonistic effects at the histamine H1 receptor [1, 2].
Benztropine (BZT) and its analogues inhibit dopamine uptake and bind with moderate to high affinity to the dopamine transporter (DAT). BZT analogues also exhibit varied binding affinities for muscarinic M(1) and histamine H(1) receptors. The BZT analogues showed a wide range of histamine H(1) receptor (K(i)=16-37,600 nM) and DAT (K(i)=8.5-6370 nM) binding affinities [3]. References: | |||||

Benztropine mesylate Dilution Calculator

Benztropine mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4781 mL | 12.3907 mL | 24.7813 mL | 49.5626 mL | 61.9533 mL |
| 5 mM | 0.4956 mL | 2.4781 mL | 4.9563 mL | 9.9125 mL | 12.3907 mL |
| 10 mM | 0.2478 mL | 1.2391 mL | 2.4781 mL | 4.9563 mL | 6.1953 mL |
| 50 mM | 0.0496 mL | 0.2478 mL | 0.4956 mL | 0.9913 mL | 1.2391 mL |
| 100 mM | 0.0248 mL | 0.1239 mL | 0.2478 mL | 0.4956 mL | 0.6195 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Benztropine is a dopamine transporter (DAT) inhibitor with IC50 of 118 nM.
- Shizukaol A
Catalog No.:BCN6984
CAS No.:131984-98-0
- 3,6,19-Trihydroxy-23-oxo-12-ursen-28-oic acid
Catalog No.:BCN1584
CAS No.:131984-82-2
- CC0651
Catalog No.:BCC4200
CAS No.:1319207-44-7
- 1,3,6,8-tetrahydroxy-4-(3-methyl-2-buten-1-yl)-9H-Xanthen-9-one
Catalog No.:BCN1585
CAS No.:1319198-98-5
- Paricalcitol
Catalog No.:BCC1839
CAS No.:131918-61-1
- Solanesol
Catalog No.:BCN2596
CAS No.:13190-97-1
- Goitrin
Catalog No.:BCN2764
CAS No.:13190-34-6
- Fudosteine
Catalog No.:BCC4661
CAS No.:13189-98-5
- 2-Hydroxyethyl Salicylate
Catalog No.:BCN3579
CAS No.:87-28-5
- (3R)-(+)-1-Benzyl-3-(tert-butoxycarbonylamino)pyrrolidine
Catalog No.:BCC8389
CAS No.:131878-23-4
- Lexacalcitol
Catalog No.:BCC1704
CAS No.:131875-08-6
- (R,R)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8396
CAS No.:131864-67-0
- Diphenylpyraline HCl
Catalog No.:BCC3768
CAS No.:132-18-3
- Pheniramine Maleate
Catalog No.:BCC4700
CAS No.:132-20-7
- Benzydamine HCl
Catalog No.:BCC4637
CAS No.:132-69-4
- Dihydrocucurbitacin B
Catalog No.:BCN3118
CAS No.:13201-14-4
- UNC 0646
Catalog No.:BCC2431
CAS No.:1320288-17-2
- UNC 0631
Catalog No.:BCC4143
CAS No.:1320288-19-4
- Cryptoacetalide
Catalog No.:BCN3139
CAS No.:132059-23-5
- Marmesin angelate
Catalog No.:BCN8139
CAS No.:13209-79-5
- Ropivacaine hydrochloride monohydrate
Catalog No.:BCC5169
CAS No.:132112-35-7
- O,O-diacetyldaurisoline
Catalog No.:BCC8221
CAS No.:132139-17-4
- Epi-Cryptoacetalide
Catalog No.:BCN3140
CAS No.:132152-57-9
- Dracoflavan A
Catalog No.:BCN3588
CAS No.:132185-42-3
New use of an old drug: inhibition of breast cancer stem cells by benztropine mesylate.[Pubmed:27894093]
Oncotarget. 2017 Jan 3;8(1):1007-1022.
Cancer stem cells (CSCs) play major roles in cancer initiation, metastasis, recurrence and therapeutic resistance. Targeting CSCs represents a promising strategy for cancer treatment. The purpose of this study was to identify selective inhibitors of breast CSCs (BCSCs). We carried out a cell-based phenotypic screening with cell viability as a primary endpoint, using a collection of 2,546 FDA-approved drugs and drug-like molecules in spheres formed by malignant human breast gland-derived cells (HMLER-shEcad cells, representing BCSCs) and control immortalized non-tumorigenic human mammary cells (HMLE cells, representing normal stem cells). 19 compounds were identified from screening. The chemically related molecules Benztropine mesylate and deptropine citrate were selected for further validation and both potently inhibited sphere formation and self-renewal of BCSCs in vitro. Benztropine mesylate treatment decreased cell subpopulations with high ALDH activity and with a CD44+/CD24- phenotype. In vivo, Benztropine mesylate inhibited tumor-initiating potential in a 4T1 mouse model. Functional studies indicated that Benztropine mesylate inhibits functions of CSCs via the acetylcholine receptors, dopamine transporters/receptors, and/or histamine receptors. In summary, our findings identify Benztropine mesylate as an inhibitor of BCSCs in vitro and in vivo. This study also provides a screening platform for identification of additional anti-CSC agents.


