GoitrinCAS# 13190-34-6 |
- Epigoitrin
Catalog No.:BCN6278
CAS No.:1072-93-1
- (S)-Goitrin
Catalog No.:BCX0834
CAS No.:500-12-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13190-34-6 | SDF | Download SDF |
| PubChem ID | 3034683 | Appearance | White powder |
| Formula | C5H7NOS | M.Wt | 129.18 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | DL-Goitrin; | ||
| Solubility | Soluble in diethyl ether | ||
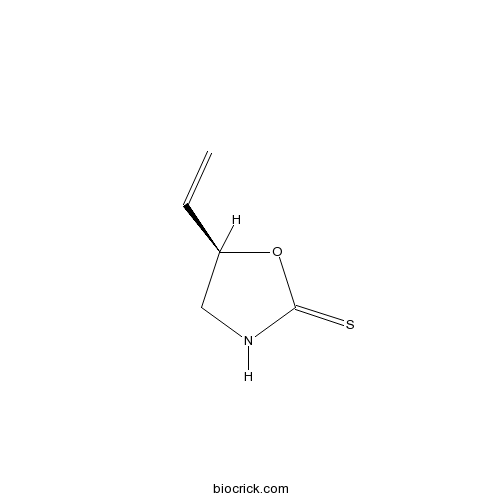
| Chemical Name | 5-ethenyl-1,3-oxazolidine-2-thione | ||
| SMILES | C=CC1CNC(=S)O1 | ||
| Standard InChIKey | UZQVYLOFLQICCT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H7NOS/c1-2-4-3-6-5(8)7-4/h2,4H,1,3H2,(H,6,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Goitrin is a potent antithyroid compound found naturally in crucifers. 2. Goitrin is responsible for the induction of glutathione S-transferases. 3. Goitrin is a moderate inhibitor of purified bovine adrenal dopamine beta-hydroxylase, leads to a depression of brain norepinephrine and to an elevation of heart and adrenal dopamine. |
| Targets | Dopamine Receptor |

Goitrin Dilution Calculator

Goitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.7411 mL | 38.7057 mL | 77.4114 mL | 154.8227 mL | 193.5284 mL |
| 5 mM | 1.5482 mL | 7.7411 mL | 15.4823 mL | 30.9645 mL | 38.7057 mL |
| 10 mM | 0.7741 mL | 3.8706 mL | 7.7411 mL | 15.4823 mL | 19.3528 mL |
| 50 mM | 0.1548 mL | 0.7741 mL | 1.5482 mL | 3.0965 mL | 3.8706 mL |
| 100 mM | 0.0774 mL | 0.3871 mL | 0.7741 mL | 1.5482 mL | 1.9353 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fudosteine
Catalog No.:BCC4661
CAS No.:13189-98-5
- 2-Hydroxyethyl Salicylate
Catalog No.:BCN3579
CAS No.:87-28-5
- (3R)-(+)-1-Benzyl-3-(tert-butoxycarbonylamino)pyrrolidine
Catalog No.:BCC8389
CAS No.:131878-23-4
- Lexacalcitol
Catalog No.:BCC1704
CAS No.:131875-08-6
- (R,R)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8396
CAS No.:131864-67-0
- Aphagranin A
Catalog No.:BCN6889
CAS No.:1318173-53-3
- Cardenolide B-1
Catalog No.:BCN4714
CAS No.:1318158-89-2
- Ugaxanthone
Catalog No.:BCN6775
CAS No.:13179-11-8
- DAU 5884 hydrochloride
Catalog No.:BCC7263
CAS No.:131780-48-8
- Flavopiridol hydrochloride
Catalog No.:BCC3925
CAS No.:131740-09-5
- Urolignoside
Catalog No.:BCN6758
CAS No.:131723-83-6
- Arbidol HCl
Catalog No.:BCC3722
CAS No.:131707-23-8
- Solanesol
Catalog No.:BCN2596
CAS No.:13190-97-1
- Paricalcitol
Catalog No.:BCC1839
CAS No.:131918-61-1
- 1,3,6,8-tetrahydroxy-4-(3-methyl-2-buten-1-yl)-9H-Xanthen-9-one
Catalog No.:BCN1585
CAS No.:1319198-98-5
- CC0651
Catalog No.:BCC4200
CAS No.:1319207-44-7
- 3,6,19-Trihydroxy-23-oxo-12-ursen-28-oic acid
Catalog No.:BCN1584
CAS No.:131984-82-2
- Shizukaol A
Catalog No.:BCN6984
CAS No.:131984-98-0
- Benztropine mesylate
Catalog No.:BCC4524
CAS No.:132-17-2
- Diphenylpyraline HCl
Catalog No.:BCC3768
CAS No.:132-18-3
- Pheniramine Maleate
Catalog No.:BCC4700
CAS No.:132-20-7
- Benzydamine HCl
Catalog No.:BCC4637
CAS No.:132-69-4
- Dihydrocucurbitacin B
Catalog No.:BCN3118
CAS No.:13201-14-4
- UNC 0646
Catalog No.:BCC2431
CAS No.:1320288-17-2
Inhibition of dopamine beta-hydroxylase by goitrin, a natural antithyroid compound.[Pubmed:2462616]
J Nat Prod. 1988 Sep-Oct;51(5):862-5.
RS-Goitrin can be conveniently prepared by a simplification of the Ettlinger procedure. Goitrin is a moderate inhibitor of purified bovine adrenal dopamine beta-hydroxylase. The administration of Goitrin leads to a depression of brain norepinephrine and to an elevation of heart and adrenal dopamine.
Glutathione S-transferase subunit induction patterns of Brussels sprouts, allyl isothiocyanate and goitrin in rat liver and small intestinal mucosa: a new approach for the identification of inducing xenobiotics.[Pubmed:2341092]
Food Chem Toxicol. 1990 Feb;28(2):81-8.
Effects of Brussels sprouts (2.5-30%), allyl isothiocyanate (0.03 and 0.1%) and Goitrin (0.02%), in the diet, on the glutathione S-transferase subunit pattern in the liver and small intestinal mucosa of male Fisher rats were investigated. A statistically significant linear relationship was found between the amount of Brussels sprouts in the diet and the induction of glutathione S-transferase subunits in two experiments. Increases in total activity of glutathione S-transferases towards 1-chloro-2,4-dinitrobenzene ranged from about 15% (2.5% Brussels sprouts in the diet) to 180% (30% Brussels sprouts in the diet) in the liver, and from 3% (2.5% Brussels sprouts) to 150% (30% Brussels sprouts) in the small intestinal mucosa. There were similar increases in the total amounts of glutathione S-transferase subunits. In the first experiment, when the average sinigrin and proGoitrin levels found in the sprouts were 1835 and 415 mumol/kg, respectively, subunit induction patterns in both the liver and the small intestinal mucosa were very similar to the pattern observed after feeding allyl isothiocyanate. In the second experiment, when the average sinigrin level found in the sprouts was as low as the proGoitrin level (both about 540 mumol/kg), a Goitrin-like induction pattern was observed. The most pronounced difference between the glutathione S-transferase subunit induction patterns due to administration of allyl isothiocyanate and Goitrin is the much stronger enhancement of subunit 2 by allyl isothiocyanate. The induction patterns of both experiments indicate that in Brussels sprouts at least two compounds, probably allyl isothiocyanate and Goitrin, are responsible for the induction of glutathione S-transferases.
Genetics and bitter taste responses to goitrin, a plant toxin found in vegetables.[Pubmed:20551074]
Chem Senses. 2010 Oct;35(8):685-92.
The perceived bitterness of cruciferous vegetables such as broccoli varies from person to person, but the functional underpinnings of this variation are not known. Some evidence suggests that it arises, in part, from variation in ability to perceive Goitrin (5-vinyloxazolidine-2-thione), a potent antithyroid compound found naturally in crucifers. Individuals vary in ability to perceive synthetic compounds similar to Goitrin, such as 6-propyl-2-thiouracil (PROP) and phenylthiocarbamide (PTC), as the result of mutations in the TAS2R38 gene, which encodes a bitter taste receptor. This suggests that taste responses to Goitrin itself may be mediated by TAS2R38. To test this hypothesis, we examined the relationships between genetic variation in TAS2R38, functional variation in the encoded receptor, and threshold taste responses to Goitrin, PROP, and PTC in 50 subjects. We found that threshold responses to Goitrin were associated with responses to both PROP (P = 8.9 x 10(-4); r(s) = 0.46) and PTC (P = 7.5 x 10(-4); r(s) = 0.46). However, functional assays revealed that Goitrin elicits a weaker response from the sensitive (PAV) allele of TAS2R38 (EC(50) = 65.0 muM) than do either PROP (EC(50) = 2.1 muM) or PTC (EC(50) = 1.1 muM) and no response at all from the insensitive (AVI) allele. Furthermore, Goitrin responses were significantly associated with mutations in TAS2R38 (P = 9.3 x 10(-3)), but the same mutations accounted for a smaller proportion of variance in Goitrin response (r(2) = 0.16) than for PROP (r(2) = 0.50) and PTC (r(2) = 0.57). These findings suggest that mutations in TAS2R38 play a role in shaping Goitrin perception, but the majority of variance must be explained by other factors.
[The occurrence of goitrogenic substances in milk. 1. Release of goitrin in the milk of cows fed on rapeseed extract cakes].[Pubmed:2416146]
Z Lebensm Unters Forsch. 1985 Nov;181(5):375-8.
A total of six cows, divided into 3 groups, were fed various amounts of rape cake containing 6 g of Goitrin/kg over a period of 7 days. The cows were milked twice a day and the Goitrin content of the heated milk samples were determined by a HPLC-method within 2 h. When rape cake was fed at 0.39, 1.9 and 3.9% resp. of the total feed this resulted in medium Goitrin values of 37, 163 and 707 micrograms/l milk. These values correspond to a transfer of about 0.1% of the original proGoitrin content in the feed. 12 h after the last rape feeding the amount of Goitrin in the milk was below the detection limit of 7 ppb. The toxicological significance of these findings are dicussed.


