CC0651E2 enzyme inhibitor CAS# 1319207-44-7 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1319207-44-7 | SDF | Download SDF |
| PubChem ID | 53239927 | Appearance | Powder |
| Formula | C20H21Cl2NO6 | M.Wt | 442.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 56 mg/mL (126.61 mM) *"≥" means soluble, but saturation unknown. | ||
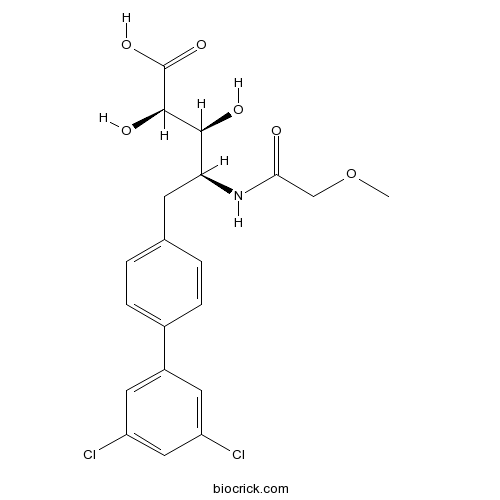
| Chemical Name | (2R,3S,4S)-5-[4-(3,5-dichlorophenyl)phenyl]-2,3-dihydroxy-4-[(2-methoxyacetyl)amino]pentanoic acid | ||
| SMILES | COCC(=O)NC(CC1=CC=C(C=C1)C2=CC(=CC(=C2)Cl)Cl)C(C(C(=O)O)O)O | ||
| Standard InChIKey | NTCBTNCWNRCBGX-YTQUADARSA-N | ||
| Standard InChI | InChI=1S/C20H21Cl2NO6/c1-29-10-17(24)23-16(18(25)19(26)20(27)28)6-11-2-4-12(5-3-11)13-7-14(21)9-15(22)8-13/h2-5,7-9,16,18-19,25-26H,6,10H2,1H3,(H,23,24)(H,27,28)/t16-,18-,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Allosteric inhibitor of human Cdc34. Inhibits hCdc34-mediated ubiquitination of p27Kip1 (IC50 = 1.72 μM). Exhibits selectivity for hCdc34 over Uba1, Ube2G1, UbcH7, UbcH5, Ube2N (Ubc13), Ube2R2, SMURF2, SspH1 and Rnf168. |

CC0651 Dilution Calculator

CC0651 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.261 mL | 11.3048 mL | 22.6096 mL | 45.2192 mL | 56.524 mL |
| 5 mM | 0.4522 mL | 2.261 mL | 4.5219 mL | 9.0438 mL | 11.3048 mL |
| 10 mM | 0.2261 mL | 1.1305 mL | 2.261 mL | 4.5219 mL | 5.6524 mL |
| 50 mM | 0.0452 mL | 0.2261 mL | 0.4522 mL | 0.9044 mL | 1.1305 mL |
| 100 mM | 0.0226 mL | 0.113 mL | 0.2261 mL | 0.4522 mL | 0.5652 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CC0651 is an allosteric inhibitor of Cdc34 ubiquitin-conjugating enzyme [1].
Ubiquitin-conjugating enzymes (E2 enzymes) are ubiquitin-carrier enzymes and perform the second step in the ubiquitin-proteasome system (UPS) that mediate the conjugation of ubiquitin to proteins for degradation. The E2 enzyme hCdc34 catalyzes the ubiquitination of proteins in conjunction with the cullin-RING (CRL) superfamily of E3 enzymes [1].
CC0651 was an allosteric inhibitor of the human Cdc34 ubiquitin-conjugating enzyme and inhibited the ubiquitination of p27Kip1 with IC50 value of 1.72 μM. In human cancer cell lines PC-3, CC0651 inhibited cell proliferation with IC50 value of 20 μM and increased the level of p27Kip1. Also, CC0651 caused production of the hCdc34-ubiquitin conjugate [1]. CC0651 trapped a weak interaction between the E2 donor ubiquitin binding site and ubiquitin. CC0651-Cdc34A-ubiquitin complex reveals that CC0651 engaged a binding pocket formed from ubiquitin and Cdc34A. Also, CC0651 suppressed the spontaneous hydrolysis rate of the ubiquitin thioester-Cdc34A [2].
References:
[1]. Ceccarelli DF, Tang X, Pelletier B, et al. An allosteric inhibitor of the human Cdc34 ubiquitin-conjugating enzyme. Cell, 2011, 145(7): 1075-1087.
[2]. Huang H, Ceccarelli DF, Orlicky S, et al. E2 enzyme inhibition by stabilization of a low-affinity interface with ubiquitin. Nat Chem Biol, 2014, 10(2): 156-163.
- 1,3,6,8-tetrahydroxy-4-(3-methyl-2-buten-1-yl)-9H-Xanthen-9-one
Catalog No.:BCN1585
CAS No.:1319198-98-5
- Paricalcitol
Catalog No.:BCC1839
CAS No.:131918-61-1
- Solanesol
Catalog No.:BCN2596
CAS No.:13190-97-1
- Goitrin
Catalog No.:BCN2764
CAS No.:13190-34-6
- Fudosteine
Catalog No.:BCC4661
CAS No.:13189-98-5
- 2-Hydroxyethyl Salicylate
Catalog No.:BCN3579
CAS No.:87-28-5
- (3R)-(+)-1-Benzyl-3-(tert-butoxycarbonylamino)pyrrolidine
Catalog No.:BCC8389
CAS No.:131878-23-4
- Lexacalcitol
Catalog No.:BCC1704
CAS No.:131875-08-6
- (R,R)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8396
CAS No.:131864-67-0
- Aphagranin A
Catalog No.:BCN6889
CAS No.:1318173-53-3
- Cardenolide B-1
Catalog No.:BCN4714
CAS No.:1318158-89-2
- Ugaxanthone
Catalog No.:BCN6775
CAS No.:13179-11-8
- 3,6,19-Trihydroxy-23-oxo-12-ursen-28-oic acid
Catalog No.:BCN1584
CAS No.:131984-82-2
- Shizukaol A
Catalog No.:BCN6984
CAS No.:131984-98-0
- Benztropine mesylate
Catalog No.:BCC4524
CAS No.:132-17-2
- Diphenylpyraline HCl
Catalog No.:BCC3768
CAS No.:132-18-3
- Pheniramine Maleate
Catalog No.:BCC4700
CAS No.:132-20-7
- Benzydamine HCl
Catalog No.:BCC4637
CAS No.:132-69-4
- Dihydrocucurbitacin B
Catalog No.:BCN3118
CAS No.:13201-14-4
- UNC 0646
Catalog No.:BCC2431
CAS No.:1320288-17-2
- UNC 0631
Catalog No.:BCC4143
CAS No.:1320288-19-4
- Cryptoacetalide
Catalog No.:BCN3139
CAS No.:132059-23-5
- Marmesin angelate
Catalog No.:BCN8139
CAS No.:13209-79-5
- Ropivacaine hydrochloride monohydrate
Catalog No.:BCC5169
CAS No.:132112-35-7
An allosteric inhibitor of the human Cdc34 ubiquitin-conjugating enzyme.[Pubmed:21683433]
Cell. 2011 Jun 24;145(7):1075-87.
In the ubiquitin-proteasome system (UPS), E2 enzymes mediate the conjugation of ubiquitin to substrates and thereby control protein stability and interactions. The E2 enzyme hCdc34 catalyzes the ubiquitination of hundreds of proteins in conjunction with the cullin-RING (CRL) superfamily of E3 enzymes. We identified a small molecule termed CC0651 that selectively inhibits hCdc34. Structure determination revealed that CC0651 inserts into a cryptic binding pocket on hCdc34 distant from the catalytic site, causing subtle but wholesale displacement of E2 secondary structural elements. CC0651 analogs inhibited proliferation of human cancer cell lines and caused accumulation of the SCF(Skp2) substrate p27(Kip1). CC0651 does not affect hCdc34 interactions with E1 or E3 enzymes or the formation of the ubiquitin thioester but instead interferes with the discharge of ubiquitin to acceptor lysine residues. E2 enzymes are thus susceptible to noncatalytic site inhibition and may represent a viable class of drug target in the UPS.
E2 enzyme inhibition by stabilization of a low-affinity interface with ubiquitin.[Pubmed:24316736]
Nat Chem Biol. 2014 Feb;10(2):156-163.
Weak protein interactions between ubiquitin and the ubiquitin-proteasome system (UPS) enzymes that mediate its covalent attachment to substrates serve to position ubiquitin for optimal catalytic transfer. We show that a small-molecule inhibitor of the E2 ubiquitin-conjugating enzyme Cdc34A, called CC0651, acts by trapping a weak interaction between ubiquitin and the E2 donor ubiquitin-binding site. A structure of the ternary CC0651-Cdc34A-ubiquitin complex reveals that the inhibitor engages a composite binding pocket formed from Cdc34A and ubiquitin. CC0651 also suppresses the spontaneous hydrolysis rate of the Cdc34A-ubiquitin thioester without decreasing the interaction between Cdc34A and the RING domain subunit of the E3 enzyme. Stabilization of the numerous other weak interactions between ubiquitin and UPS enzymes by small molecules may be a feasible strategy to selectively inhibit different UPS activities.


