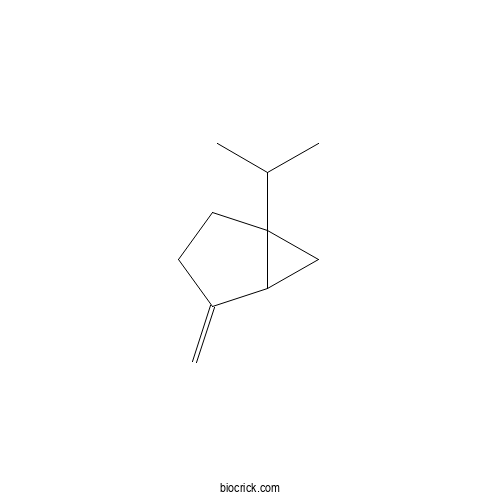
SabineneCAS# 3387-41-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3387-41-5 | SDF | Download SDF |
| PubChem ID | 18818 | Appearance | Colorless liquid |
| Formula | C10H16 | M.Wt | 136.23 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Synonyms | 4(10)-Thujene | ||
| Solubility | Freely soluble in chloroform; soluble in ethanol and methan | ||
| Chemical Name | 4-methylidene-1-propan-2-ylbicyclo[3.1.0]hexane | ||
| SMILES | CC(C)C12CCC(=C)C1C2 | ||
| Standard InChIKey | NDVASEGYNIMXJL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H16/c1-7(2)10-5-4-8(3)9(10)6-10/h7,9H,3-6H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Sabinene Dilution Calculator

Sabinene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3405 mL | 36.7026 mL | 73.4053 mL | 146.8105 mL | 183.5132 mL |
| 5 mM | 1.4681 mL | 7.3405 mL | 14.6811 mL | 29.3621 mL | 36.7026 mL |
| 10 mM | 0.7341 mL | 3.6703 mL | 7.3405 mL | 14.6811 mL | 18.3513 mL |
| 50 mM | 0.1468 mL | 0.7341 mL | 1.4681 mL | 2.9362 mL | 3.6703 mL |
| 100 mM | 0.0734 mL | 0.367 mL | 0.7341 mL | 1.4681 mL | 1.8351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rebaudioside O
Catalog No.:BCN0341
CAS No.:1220616-48-7
- Rebaudioside I
Catalog No.:BCN0340
CAS No.:1220616-34-1
- Sophoraflavanone B
Catalog No.:BCN0339
CAS No.:68682-02-0
- (S)-4',5,7-Trihydroxy-6-prenylflavanone
Catalog No.:BCN0338
CAS No.:68682-01-9
- Pinocembroside
Catalog No.:BCN0337
CAS No.:75829-43-5
- Naringenin chalcone
Catalog No.:BCN0336
CAS No.:25515-46-2
- 16-O-Methylcafestol
Catalog No.:BCN0335
CAS No.:108214-28-4
- 6-Methoxytricin
Catalog No.:BCN0334
CAS No.:76015-42-4
- (+)-Lupanine hydrochloride
Catalog No.:BCN0333
CAS No.:1025-39-4
- Lavandulyl acetate
Catalog No.:BCN0332
CAS No.:25905-14-0
- Lavandulol
Catalog No.:BCN0331
CAS No.:58461-27-1
- Lactucin
Catalog No.:BCN0330
CAS No.:1891-29-8
- (RS)-Sakuranetin
Catalog No.:BCN0343
CAS No.:520-29-6
- trans-Sinapic acid
Catalog No.:BCN0344
CAS No.:7362-37-0
- Solanthrene
Catalog No.:BCN0345
CAS No.:26516-51-8
- Cannabisin G
Catalog No.:BCN0346
CAS No.:
- Tabersonine hydrochloride
Catalog No.:BCN0347
CAS No.:29479-00-3
- Theacrine
Catalog No.:BCN0348
CAS No.:2309-49-1
- α,β-Thujone
Catalog No.:BCN0349
CAS No.:76231-76-0
- Tropine
Catalog No.:BCN0350
CAS No.:120-29-6
- (+/-)-Praeruptorin B
Catalog No.:BCN0351
CAS No.:73069-26-8
- Echiumine N-oxide
Catalog No.:BCN0352
CAS No.:685554-68-1
- 7-Acetylintermedine N-oxide
Catalog No.:BCN0353
CAS No.:685132-59-6
- Sinapine chloride
Catalog No.:BCN0354
CAS No.:6484-80-6
Not Only a Weed Plant-Biological Activities of Essential Oil and Hydrosol of Dittrichia viscosa (L.) Greuter.[Pubmed:34579370]
Plants (Basel). 2021 Sep 4;10(9). pii: plants10091837.
With the increasing interest in obtaining biologically active compounds from natural sources, Dittrichia viscosa (L.) Greuter (Asteraceae) came into our focus as a readily available and aromatic wild shrub widely distributed in the Mediterranean region. This work provides a phytochemical profile of D. viscosa in terms of parallel chemical composition in the lipophilic fraction (essential oil) and the water fraction (hydrosol). GC-MS analysis identified 1,8-cineole, caryophyllene oxide, alpha-terpenyl acetate, and alpha-muurolol as the major components of the essential oil, while in the hydrosol p-menth-1-en-9-ol, 1,8-cineole, linalool, cis-Sabinene hydrate, and alpha-muurolol were the major volatile components. 3,4-Dihydroxybenzoic acid was found to be the predominant compound in the hydrosol composition by HPLC analysis. The antimicrobial potential of both extracts was evaluated against thirteen opportunistic pathogens associated with common skin and wound infections and emerging food spoilage microorganisms. The antimicrobial activity of the essential oil suggests that the volatiles of D. viscosa could be used as novel antimicrobial agents. The antiproliferative results of D. viscosa volatiles are also new findings, which showed promising activity against three cancer cell lines: HeLa (cervical cancer cell line), HCT116 (human colon cancer cell line), and U2OS (human osteosarcoma cell line). The decrease in GSH level observed in hydrosol-treated HeLa cells suggests oxidative stress as a possible mechanism of the antiproliferative effect of hydrosol on tumor cells. The presented results are also the first report of significant antiphytoviral activity of hydrosol against tobacco mosaic virus (TMV) infection. Based on the results, D. viscosa might have the potential to be used in crop protection, as a natural disinfectant and natural anticancer agent.
Chemical composition and antimicrobial activity of the leaf essential oil of Ravenia spectabilis Engl. (Rutaceae).[Pubmed:34570617]
Nat Prod Res. 2021 Sep 27:1-4.
Ravenia spectabilis Engl. belongs to the family Rutaceae is known to possess several biologically active phytomolecules. This study was planned to investigate the chemical composition and antimicrobial activity of the leaf essential oil of R. spectabilis. The hydrodistillation of fresh leaves of R. spectabilis gave 0.19 +/- 0.02% essential oil. The resulting essential oil was analysed by gas chromatography-flame ionization detector (GC-FID) and gas chromatography-mass spectrometry (GC-MS). Altogether, thirty-one constituents forming 97.6 +/- 1.72% of the total oil composition were identified. Major components of the oil were Sabinene (60.8 +/- 0.36%), alpha-pinene (5.4 +/- 0.30%), myrcene (4.8 +/- 0.25%), delta-3-carene (4.7 +/- 0.62%) and beta-pinene (4.3 +/- 0.17%). The in-vitro antimicrobial potential of the oil was examined against eight human pathogenic bacterial and fungal strains. The essential oil showed significant activity against Staphylococcus aureus, Staphylococcus epidermidis, methicillin-resistant Staphylococcus aureus, methicillin-resistant Staphylococcus epidermidis, Candida albicans, and Candida kefyr. This is the first report on R. spectabilis leaf essential oil composition and its antimicrobial activity. The essential oil could be a promising natural source of Sabinene and antimicrobial for developing new phytotherapeutics.
Quality Characteristics and Antioxidant Potential of Lemon (Citrus limon Burm. f.) Seed Oil Extracted by Different Methods.[Pubmed:34568400]
Front Nutr. 2021 Sep 9;8:644406.
Lemon (Citrus limon Burm. f.) is one of the most widely produced and consumed fruits in the world. The seeds of lemon are generally discarded as waste. The purpose of this study was to investigate the quality characteristics and antioxidant potential of lemon seed oil obtained by four extraction methods (roasted-pressing at 170 degrees C, RP-170; roasted-pressing at 100 degrees C, RP-100; cold-pressing, CP; and supercritical fluid, SF). No significant differences in the viscosity, density, and refractive index were observed in the oil obtained from different methods. In the case of Hunter's value, L (lightness) and b (yellowness) values of SF were higher than those of the others. The oil obtained by the CP method exhibited higher levels of Ca (252.17 mg/kg), Cu (2.38 mg/kg), K (225.98 mg/kg), and Mo (0.47 mg/kg) than that of other methods. The highest contents of total phenols (165.90 mg/mL) and flavonoids (21.69 mg/mL) were significantly high in oil obtained by the SF method. Oleic and linoleic acids consisted of principal fatty acids, which were significantly higher in oil obtained by RP-170. Higher amounts of volatile flavor compounds, such as gamma-terpinene, Sabinene, and limonene, were observed in CP compared to those observed for the other methods. This study elucidates the effects of different methods of oil extraction on the composition of lemon seed oil and highlights potential applications of these benefits in the food, cosmetic, pharmaceutical, and/or fragrance industries.
Chemical composition and chiral analysis of beta-myrcene rich essential oil from Glossocardia bosvallia (L.f.) DC.[Pubmed:34542366]
Nat Prod Res. 2021 Sep 20:1-4.
Essential oil from the aerial parts and flowers of Glossocardia bosvallia (L.f.) DC. was analysed using GC, chiral GC, GC-MS and NMR spectroscopy. Chemical composition and identification of compounds in the essential oils were determined by GC and GC-MS analysis respectively. GC analysis depicted beta-myrcene as the major compound in both the essential oils, aerial parts containing 61.59% and flowers constitutes 44.86%. Occurrence of beta-myrcene was also confirmed using (1)H, (13)C and DEPT 135 degrees NMR spectroscopy. Chiral GC illustrated the enantiomeric composition of monoterpene hydrocarbons, in which (1S)-(-)-beta-pinene, (-)-Sabinene and (4S)-(-)-limonene were the major enantiomers. The present study reports the essential oil composition and enantiomeric composition of beta-myrcene rich G. bosvallia essential oil for the first time. G. bosvallia essential oil can be a good source of beta-myrcene, a compound with wide range of biological properties.
Chemical composition, antioxidant and anticholinesterase activity of the essential oil of algerian cachrys sicula L.[Pubmed:34507514]
Nat Prod Res. 2021 Sep 10:1-9.
In this work, in order to explore a new Algerian medicinal plant used in traditional medicine, the essential oil of the leaves of Cachrys sicula L. (Apiaceae) collected from Algeria, obtained by hydrodistillation, was analyzed by GC/MS. Thirty-two compounds were identified accounting for 98.6% of the total oil, which is characterized by a high content of monoterpene hydrocarbons (74.8%). The main constituents of the essential oil were beta-pinene (17.9%), Sabinene (17.8%), myrcene (12%), and alpha-pinene (11.4%). In vitro antioxidant activity of the essential oil was assayed by three methods, namely ABTS(*+), metal chelating, and DPPH(*) assays. The antioxidant activity of the oil was higher in the ABTS(*+) method. Anticholinesterase activity was screened by the Ellman method against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) which are the chief enzymes of Alzheimer's disease. The results showed mild acetylcholinesterase and good butyrylcholinesterase inhibitory effects.
Chemical and Antimicrobial Analyses of Juniperus chinensis and Juniperus seravschanica Essential Oils and Comparison with Their Methanolic Crude Extracts.[Pubmed:34497647]
Int J Anal Chem. 2021 Aug 30;2021:9937522.
Juniperus chinensis and Juniperus seravschanica are commonly used in the traditional folk medicine to treat microbial infection. In this study, the essential oils obtained from the leaves of J. chinensis growing in Malaysia and J. seravschanica growing in Oman were analysed by head space-solid phase microextraction-gas chromatography mass spectrometry (HS-SPME-GC-MS) and screened for antimicrobial activities against Escherichia coli (NCTC 10418), Pseudomonas aeruginosa (NCTC 10662), Bacillus subtilis ATCC6059, Micrococcus luteus (ATCC 9341), Staphylococcus aureus (NCTC 6571), and methicillin-resistant S. aureus (MRSA; ATCC 33591). To compare the antimicrobial activities of extracts using different extraction methods, methanol extraction was performed to obtain crude extracts from the leaves of J. chinensis and J. seravschanica for antimicrobial analysis. The HS-SPME-GS-MS analysis of the essential oils from the leaves of J. chinensis and J. seravschanica identified 37 and 36 components, respectively. Essential oils from these two species had distinctive chemical component profiles, with alpha-pinene (27.2%) as the major component of J. chinensis essential oil, while dl-limonene (45.2%) constitutes the major component of J. seravschanica essential oil. Essential oils of these two species shared only six similar terpenoids compounds: alpha-pinene, beta-pinene, gamma-elemene, Sabinene, elemol, and 3-cyclohexen-1-ol. Overall, the essential oils showed antimicrobial activities against all the six bacterial strains tested, with the highest antagonistic activity against M. luteus and B. cereus; while, methanolic crude extracts showed the highest activities against S. aureus and MRSA strains. The methanolic crude extracts demonstrated significantly higher antibacterial activity against the Gram-positive bacteria (p < 0.005); while, the essential oils of Juniperus did not show significant differences between Gram-positive and Gram-negative bacteria. Future studies are needed to investigate the active compounds present in the essential oils and methanolic crude extracts that confer the selectivity in the antimicrobial activity.
Identification of a wild carrot as carrot psylla (Bactericera trigonica) attractant and host plant chemistry.[Pubmed:34482913]
Plant Sci. 2021 Oct;311:111011.
Carrot psylla is one of the devastating pests of carrot throughout northern Europe and the Mediterranean basin. Here we characterized the behavioral response of psylla females towards different carrot germplasm and identified the chemical cues involved in the host selection of psylla females by oviposition choice experiments and metabolic profiling of leaf volatiles. In choice assays, carrot psylla displayed differential responses to tested 14 germplasm. Among germplasm, wild accessions 21793 and 20465 were highly preferred by carrot psylla, while wild accessions 20465 and the orange cultivar Nairobi were less. In non-choice experiments conducted only with this four-germplasm revealed that the carrot psylla females gave higher preference to the Nairobi and wild accession 20465, indicating the vicinity to other host plants in the same area might affect female preference. Moreover, the nymph development and survival experiments showed the lowest nymphs survival rate on the wild accessions 21793 and 20497. Furthermore, the volatile emissions among different carrot cultivars infested with psylla showed qualitative and quantitative differences versus intact plants. Among these volatiles, apiol, beta-asarone, myristicin, and Sabinene showed a relationship with psyllas growth and survival. We also showed that myristicin and Sabinene exogenous applications caused a dramatic reduction in the number of eggs laid by psylla and subsequent nymph survival. This is an initial study of the volatiles that mediate attraction and oviposition preference of carrot psylla in response to its host plant. The results from this study provide baseline information for the development of new control strategies against carrot psylla.
In vitro acaricidal activity of essential oil and crude extracts of Laurus nobilis, (Lauraceae) grown in Tunisia, against arthropod ectoparasites of livestock and poultry: Hyalomma scupense and Dermanyssus gallinae.[Pubmed:34388421]
Vet Parasitol. 2021 Oct;298:109507.
The current study assayed the toxicity of Laurus nobilis essential oil and crude extracts obtained using solvents of increasing polarity (cyclohexane, acetone and ethanol), on two ectoparasites of veterinary importance, i.e., Hyalomma scupense and Dermanyssus gallinae. The major components detected in bay laurel essential oil were dominated by 1.8-cineole (46.56 %), alpha-terpinenyl acetate (13.99 %), Sabinene (7.69), alpha-pinene (5.75), linalool (5.50), methyleugenol (5.36 %) and beta-pinene (3.97). The highest total phenolic and flavonoids contents were present in the ethalonic extract of L. nobilis leaves at an amount of 152.88 mg gallic acid equivalents per gram of dry weight (GAE/g DW) and 21.77 mg quercetin equivalent per gram of dry weight (QE/g DW), respectively. In vitro acaricidal effects of essensial oil and crude extract of L. nobilis against H. scupense were ascertained by adult immersion test of engorged females (AIT) and larval packet test (LPT) compared with a reference drug amitraz. The essential oil exhibited strong acaricidal activity against tick engorged female and inhibition of hatching eggs. After 24 h of exposure, at the highest tested concentration (100 mg/mL) essential oil induced 90.67 % mortality of H. scupense larvae (LC50 = 10.69 mg/mL). Otherwise, essential oil exhibited high acaricidal activity compared to extracts, and among the extract, the ethanolic extract revealed the highest acaricidal efficacy (81.27 % female mortality). Results from mite contact toxicity showed that essential oil and extracts from L. nobilis were toxic to D. gallinae. Bay essential oil was both more toxic to mites, and faster in exerting this toxicity than other tested crude extracts. L. nobilis essential oil concentration leaded to enhance mortality of D. gallinae reaching the highest (100 %) mortality at 12 h with a concentration of 320 mg/mL. While, ethanolic extract acheived this rate after 24 h of exposure at same concentration. Cyclohexanic extract showed weak acaricidal activity.
Identification and Behavioral Assays of Alarm Pheromone in the Vetch Aphid Megoura viciae.[Pubmed:34347235]
J Chem Ecol. 2021 Sep;47(8-9):740-746.
Aphids are destructive pests, and alarm pheromones play a key role in their chemical ecology. Here, we conducted a detailed analysis of terpenoids in the vetch aphid, Megoura viciae, and its host plant Pisum sativum using gas chromatography/mass spectrometry. Four major components, (-)-beta-pinene (49.74%), (E)-beta-farnesene (32.64%), (-)-alpha-pinene (9.42%) and ( +)-limonene (5.24%), along with trace amounts of ( +)-Sabinene, camphene and alpha-terpineol) (3.14%) were found in the aphid. In contrast, few terpenoids were found in the host plant, consisting mainly of squalene (66.13%) and its analog 2,3-epoxysqualene (31.59%). Quantitative analysis of the four major terpenes in different developmental stages of the aphid showed that amounts of the monoterpenes increased with increasing stage, while the sesquiterpene amount peaked in the 3(rd) instar. (-)-beta-Pinene was the most abundant terpene at all developmental stages. Behavioral assays using a three-compartment olfactometer revealed that the repellency of single compounds varied in a concentration-dependent manner, but two mixtures [(-)-alpha-pinene: (-)-beta-pinene: (E)-beta-farnesene: ( +)-limonene = 1:44.4:6.5:2.2 or 1:18.4:1.3:0.8], were repellent at all concentrations tested. Our results suggest that (-)-alpha-pinene and (-)-beta-pinene are the major active components of the alarm pheromone of M. viciae, but that mixtures play a key role in the alarm response. Our study contributes to the understanding of the chemical ecology of aphids and may help design new control strategies against this aphid pest.
Effective Extraction of Limonene and Hibaene from Hinoki (Chamaecyparis obtusa) Using Ionic Liquid and Deep Eutectic Solvent.[Pubmed:34299543]
Molecules. 2021 Jul 14;26(14). pii: molecules26144271.
The essential oils of hinoki (Chamaecyparis obtusa) leaves have anti-bacterial, anti-fungal, and relaxation properties that are likely associated with the major components such as Sabinene, alpha-terpinyl acetate, limonene, elemol, myrcene, and hibaene. The present study describes the use of a cellulose-dissolving ionic liquid (IL) [C2mim][(MeO)(H)PO2] and low-toxicity solvents called betaine-based deep eutectic solvents (DESs) for the efficient extraction of hinoki essential oils. As a control method, organic solvent extraction was performed using either hexane, ethyl acetate (EtOAc), or acetone at 30 degrees C for 1 h. Both the experimental and control methods were conducted under the same conditions, which relied on partial dissolution of the leaves using the IL and DESs before partitioning the hinoki oils into the organic solvent for analysis. Quantitative analysis was performed using gas chromatography-mass spectrometry (GC-MS) in selected ion monitoring (SIM) mode. The results indicated that extraction using the [C2mim][(MeO)(H)PO2]/acetone bilayer system improved the yields of limonene and hibaene, 1.5- and 1.9-fold, respectively, when compared with the control method. In addition, extraction using betaine/l-lactic acid (molar ratio 1:1) gave the greatest yields for both limonene and hibaene, 1.3-fold and 1.5-fold greater, respectively, than when using an organic solvent. These results demonstrate the effective extraction of essential oils from plant leaves under conditions milder than those needed for the conventional method. The less toxic and environmentally begin DESs for the extraction are also applicable to the food and cosmetic industries.
Antioxidant Activity and Molecular Docking Study of Volatile Constituents from Different Aromatic Lamiaceous Plants Cultivated in Madinah Monawara, Saudi Arabia.[Pubmed:34299420]
Molecules. 2021 Jul 7;26(14). pii: molecules26144145.
A comparative study of volatile constituents, antioxidant activity, and molecular docking was conducted between essential oils from Mentha longifolia L., Mentha spicata L., and Origanum majorana L., widely cultivated in Madinah. The investigation of volatile oils extracted by hydrodistillation was performed using Gas Chromatography-Mass Spectrometry (GC-MS). A total number of 29, 42, and 29 components were identified in M. longifolia, M. spicata, and O. majorana representing, respectively, 95.91, 94.62, and 98.42, of the total oils. Pulegone (38.42%), 1,8-cineole (15.60%), menthone (13.20%), and isopulegone (9.81%) were the dominant compounds in M. longifolia oil; carvone (35.14%), limonene (27.11%), germacrene D (4.73%), and beta-caryophyllene (3.02%) were dominant in M. spicata oil; terpin-4-ol (42.47%), trans-Sabinene hydrate (8.52%), gamma-terpinene (7.90%), alpha-terpineol (7.38%), linalool (6.35%), alpha-terpinene (5.42%), and cis-Sabinene hydrate (3.14%) were dominant in O. majorana oil. The antioxidant activity, assessed using DPPH free radical-scavenging and ABTS assays, was found to be the highest in O. majorana volatile oil, followed by M. spicata and M. longifolia, which is consistent with the differences in total phenolic content and volatile constituents identified in investigated oils. In the same context, molecular docking of the main identified volatiles on NADPH oxidase showed a higher binding affinity for cis-verbenyl acetate, followed by beta-elemene and linalool, compared to the control (dextromethorphan). These results prove significant antioxidant abilities of the investigated oils, which may be considered for further analyses concerning the control of oxidative stress, as well as for their use as possible antioxidant agents in the pharmaceutical industry.
A nanoemulsion-based nanogel of Citrus limon essential oil with leishmanicidal activity against Leishmania tropica and Leishmania major.[Pubmed:34295043]
J Parasit Dis. 2021 Jun;45(2):441-448.
Cutaneous leishmaniasis is one of the diseases that severely affects human skin. Nanogels are the well-known formulation for topical drug delivery due to easy usage, high loading capacity, and physical and chemical stabilities. In this study, the toxicity effect of three essential oils, including Mentha piperita, Anethum graveolens, and Citrus limon (CLEO), was evaluated against Leishmania major and Leishmania tropica. Ingredients of CLEO as the most potent essential oil were identified using GC-MS analysis. The five major components were limonene (61.83%), Sabinene (16.99%), trans-limonene oxide (3.08%), cis-limonene oxide (2.27%), and 1,2-cyclohexane diol, 1-methyl-4-(1-methylethenyl) (1.50%). The nanogel of CLEO (CLNgel) was prepared by the addition of carbomer 940 (1% w/v) to the prepared nanoemulsion with a droplet size of 146 +/- 12 nm. The viscosity of CLNgel was fitted with a regression of non-Newtonian materials, Carreau-Yasuda. Interestingly, CLNgel at a concentration of 80 microg/mL reduced the viability of both species to 0%. Therefore, the prepared prototype can/could/would be used as an excellent nanoformulation for in vivo studies.
Black cardamom essential oil prevents Escherichia coli O157:H7 and Salmonella Typhimurium JSG 1748 biofilm formation through inhibition of quorum sensing.[Pubmed:34294980]
J Food Sci Technol. 2021 Aug;58(8):3183-3191.
This study aimed to investigate the chemical composition, using GC-MS, and anti-biofilm potential of black cardamom essential oil (BCEO) against biofilms of Escherichia coli O157:H7 and Salmonella Typhimurium JSG 1748 through inhibition of bacterial quorum sensing. GC-MS quantification demonstrated that BCEO contains 1,8-cineole (44.24%), alpha-terpinyl acetate (12.25%), nerolidol (6.03%), and Sabinene (5.96%) as the major bioactive compounds. Antioxidant assays for BCEO revealed the total phenolic and flavonoid mean values were 1325.03 +/- 7.69 mg GAE 100/g and 168.25 +/- 5.26 mg CE/g, respectively. In regards to antimicrobial potential, Candida albicans was the most sensitive species compared to Streptococcus mutans, Staphylococcus aureus, Listeria monocytogenes, Bacillus cereus, and Salmonella Typhimurium with the following zones of inhibition; 14.4 +/- 0.52, 13.2 +/- 0.42, 11.2 +/- 0.28, 11.0 +/- 0.52, 8.2 +/- 0.24 and 6.6 +/- 0.18 mm in diameter, respectively. Biofilm inhibition by BCEO was concentration-dependent, when various concentrations of 0.03, 0.06, 0.12, 0.25 and 0.5% were applied, 33.67, 34.14, 38.66, 46.65 and 50.17% of Salmonella Typhimurium biofilm was inhibited, while 47.31, 54.15, 76.57, 83.36 and 84.63% of Escherichia coli biofilm formation was prevented. Chromobacterium violaceum ATCC 12,472 and its product violacein, was used as a microbial indicator for enhancement or inhibition of quorum sensing. Our data showed that 0.5% of BCEO inhibited violacein production without influencing the growth of Chromobacterium violaceum, while 1% of BCEO, caused 100% inhibtion of violacein production together with 30% inhibition of growth. This study shows that BCEO possesses promising antioxidant and antimicrobial potential, and found anti-biofilm activities linked to the quenching of the quorum sensing system of E. coli and S. Typhimurium.
Identification of Chemotypic Markers in Three Chemotype Categories of Cannabis Using Secondary Metabolites Profiled in Inflorescences, Leaves, Stem Bark, and Roots.[Pubmed:34276749]
Front Plant Sci. 2021 Jul 1;12:699530.
Previous chemotaxonomic studies of cannabis only focused on tetrahydrocannabinol (THC) dominant strains while excluded the cannabidiol (CBD) dominant strains and intermediate strains (THC approximately CBD). This study investigated the utility of the full spectrum of secondary metabolites in different plant parts in three cannabis chemotypes (THC dominant, intermediate, and CBD dominant) for chemotaxonomic discrimination. Hierarchical clustering, principal component analysis (PCA), and canonical correlation analysis assigned 21 cannabis varieties into three chemotypes using the content and ratio of cannabinoids, terpenoids, flavonoids, sterols, and triterpenoids across inflorescences, leaves, stem bark, and roots. The same clustering results were obtained using secondary metabolites, omitting THC and CBD. Significant chemical differences were identified in these three chemotypes. Cannabinoids, terpenoids, flavonoids had differentiation power while sterols and triterpenoids had none. CBD dominant strains had higher amounts of total CBD, cannabidivarin (CBDV), cannabichromene (CBC), alpha-pinene, beta-myrcene, (-)-guaiol, beta-eudesmol, alpha-eudesmol, alpha-bisabolol, orientin, vitexin, and isovitexin, while THC dominant strains had higher total THC, total tetrahydrocannabivarin (THCV), total cannabigerol (CBG), camphene, limonene, ocimene, Sabinene hydrate, terpinolene, linalool, fenchol, alpha-terpineol, beta-caryophyllene, trans-beta-farnesene, alpha-humulene, trans-nerolidol, quercetin, and kaempferol. Compound levels in intermediate strains were generally equal to or in between those in CBD dominant and THC dominant strains. Overall, with higher amounts of beta-myrcene, (-)-guaiol, beta-eudesmol, alpha-eudesmol, and alpha-bisabolol, intermediate strains more resemble CBD dominant strains than THC dominant strains. The results of this study provide a comprehensive profile of bioactive compounds in three chemotypes for medical purposes. The simultaneous presence of a predominant number of identified chemotype markers (with or without THC and CBD) could be used as chemical fingerprints for quality standardization or strain identification for research, clinical studies, and cannabis product manufacturing.
Changes in volatiles and aroma profile of andaliman (Zanthoxylum acanthopodium DC.) upon various drying techniques.[Pubmed:34237576]
Food Chem. 2021 Dec 15;365:130483.
Andaliman is a highly perishable Indonesian spice that could be preserved by drying. As the drying influence on andaliman volatiles and aroma profile has not been reported, this study aimed to determine the impact of five drying processes on them and the critical volatiles correlated with favorable aroma attributes. Limonene, geranial, alpha-pinene, Sabinene, beta-myrcene, (E)-2-hexenal tended to decrease while geranyl acetate, citronellal, neral tended to increase upon drying. Limonene, andaliman major volatiles, was lost considerably from 28093 microg/g solids (fresh) to 19299-21857 microg/g solids (dried). Drying significantly altered citrus, orange peel, green, warm, and lime leaf aroma. Citronellal, limonene, (Z)-beta-ocimene, (E)-beta-ocimene, beta-citronellol, Sabinene, and geranial, played substantial roles in andaliman sensory acceptability due to significant correlation to the favorable aroma attributes (citrus, orange peel, acidic). Oven drying was proposed as the ideal drying method because of its short duration, low water activity, superior overall liking, and high volatile content.


