Naringenin chalconeCAS# 25515-46-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25515-46-2 | SDF | Download SDF |
| PubChem ID | 5280960 | Appearance | Orange powder |
| Formula | C15H12O5 | M.Wt | 272.25 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 73692-50-9;Chalconaringenin; Chalcononaringenin; Isosalipurpol; trans-2',4,4',6'-Tetrahydroxychalcone | ||
| Solubility | Soluble in methan | ||
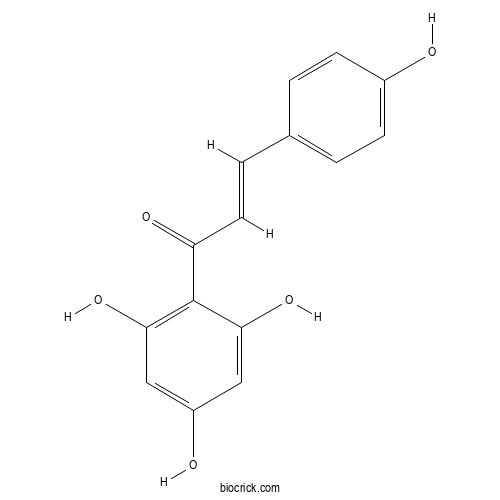
| Chemical Name | (E)-3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)prop-2-en-1-one | ||
| SMILES | C1=CC(=CC=C1C=CC(=O)C2=C(C=C(C=C2O)O)O)O | ||
| Standard InChIKey | YQHMWTPYORBCMF-ZZXKWVIFSA-N | ||
| Standard InChI | InChI=1S/C15H12O5/c16-10-4-1-9(2-5-10)3-6-12(18)15-13(19)7-11(17)8-14(15)20/h1-8,16-17,19-20H/b6-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Naringenin chalcone Dilution Calculator

Naringenin chalcone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6731 mL | 18.3655 mL | 36.7309 mL | 73.4619 mL | 91.8274 mL |
| 5 mM | 0.7346 mL | 3.6731 mL | 7.3462 mL | 14.6924 mL | 18.3655 mL |
| 10 mM | 0.3673 mL | 1.8365 mL | 3.6731 mL | 7.3462 mL | 9.1827 mL |
| 50 mM | 0.0735 mL | 0.3673 mL | 0.7346 mL | 1.4692 mL | 1.8365 mL |
| 100 mM | 0.0367 mL | 0.1837 mL | 0.3673 mL | 0.7346 mL | 0.9183 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 16-O-Methylcafestol
Catalog No.:BCN0335
CAS No.:108214-28-4
- 6-Methoxytricin
Catalog No.:BCN0334
CAS No.:76015-42-4
- (+)-Lupanine hydrochloride
Catalog No.:BCN0333
CAS No.:1025-39-4
- Lavandulyl acetate
Catalog No.:BCN0332
CAS No.:25905-14-0
- Lavandulol
Catalog No.:BCN0331
CAS No.:58461-27-1
- Lactucin
Catalog No.:BCN0330
CAS No.:1891-29-8
- Isoxanthohumol
Catalog No.:BCN0329
CAS No.:521-48-2
- Isomenthone
Catalog No.:BCN0328
CAS No.:491-07-6
- Hyperforin (stable Dicyclohexylammonium salt)
Catalog No.:BCN0327
CAS No.:238074-03-8
- 3'-Hydroxyflavone
Catalog No.:BCN0326
CAS No.:70460-18-3
- (Rac)-Hesperetin
Catalog No.:BCN0325
CAS No.:69097-99-0
- Grindelic acid
Catalog No.:BCN0324
CAS No.:1438-57-9
- Pinocembroside
Catalog No.:BCN0337
CAS No.:75829-43-5
- (S)-4',5,7-Trihydroxy-6-prenylflavanone
Catalog No.:BCN0338
CAS No.:68682-01-9
- Sophoraflavanone B
Catalog No.:BCN0339
CAS No.:68682-02-0
- Rebaudioside I
Catalog No.:BCN0340
CAS No.:1220616-34-1
- Rebaudioside O
Catalog No.:BCN0341
CAS No.:1220616-48-7
- Sabinene
Catalog No.:BCN0342
CAS No.:3387-41-5
- (RS)-Sakuranetin
Catalog No.:BCN0343
CAS No.:520-29-6
- trans-Sinapic acid
Catalog No.:BCN0344
CAS No.:7362-37-0
- Solanthrene
Catalog No.:BCN0345
CAS No.:26516-51-8
- Cannabisin G
Catalog No.:BCN0346
CAS No.:
- Tabersonine hydrochloride
Catalog No.:BCN0347
CAS No.:29479-00-3
- Theacrine
Catalog No.:BCN0348
CAS No.:2309-49-1
Scrapped but not neglected: Insights into the composition, molecular modulation and antioxidant capacity of phenols in peel of eggplant (Solanum melongena L.) fruits at different developmental stages.[Pubmed:34488153]
Plant Physiol Biochem. 2021 Aug 24;167:678-690.
Eggplant fruits are normally harvested and marketed when they reach the commercial maturity, that precedes the physiological ripening when dramatic changes in taste, composition and peel color take place. The biochemical changes in fruit peel across the developmental stages, characterized also by a sizeable decrement of anthocyanins, were studied in four eggplant genotypes differing for fruit pigmentation. HPLC-DAD, HPLC-ESI-MS and NMR analyses identified Naringenin chalcone and naringenin 7-O-glucoside as the main phenolic compounds in extracts from the physiological ripe stage, along with compounds tentatively identified as glycosylated Naringenin chalcone, naringenin and kaempferol. On average, the levels of anthocyanins, responsible for the peel pigmentation, dropped by 75% during development, while, surprisingly, the level of total phenols showed a slight decrease of 16%, with a final concentration of more than 1000 mg/100g dw. RT-qPCR expression profiling of nine genes coding for enzymes putatively acting at different steps of the involved pathways showed modulation mostly consistent with the observed changes in phenolic composition, with a remarkable decrease in the activity of flavonol reductase and an increase in flavonol synthase during berry development. Antioxidant activity monitored by peroxyl scavenging was similar at all developmental stages while Fremy's analysis evidenced a slight decrement at full physiological ripening. These results are valuable to address the improvement of eggplant commercial fruit quality and the valorization of unmarketable physiological ripe fruits, especially for the newly accumulation of the health-promoting compounds chalcones and flavanones.
Structure, isomerization and dimerization processes of naringenin flavonoids.[Pubmed:34388230]
Phys Chem Chem Phys. 2021 Sep 7;23(33):18068-18077.
In this study, the theoretical and experimental results on the molecular structure and reactivity of the plant flavonoids Naringenin chalcone and naringenin are reported. UV-vis and Raman spectra were recorded and their main bands have been assigned theoretically. Moreover, the analysis of the Naringenin chalcone-naringenin cyclization-isomerization reaction and the formation of homodimers and heterodimers have been performed within a DFT framework. The presence of H-bonded water networks is mandatory to make the cyclization energetically suitable, suggesting that this equilibrium will occur in an aqueous intracellular environment rather than in the extracellular and hydrophobic plant cuticles. Additionally, the preferential formation of homodimers stabilized by pi-pi stacking that will interact with other dimers by H-bonding over the formation of Naringenin chalcone-naringenin heterodimers is also proposed in a hydrophobic environment. These results give a plausible model to explain how flavonoids are located within the cuticle molecular arrangement.
New Phenolic Compounds in Posidonia oceanica Seagrass: A Comprehensive Array Using High Resolution Mass Spectrometry.[Pubmed:33923075]
Plants (Basel). 2021 Apr 25;10(5). pii: plants10050864.
The studies on the Posidonia oceanica Delile (P. oceanica) phenolic composition have been focused on the foliar tissues and have often neglected the phenolic compounds in rhizomes or roots alike. With the current improvements in high resolution mass spectrometry (HRMS) analyzers, such as the Orbitrap MS, there is a new opportunity to more deeply study P. oceanica. One of the benefits is the possibility of conducting an exhaustive phenolic monitoring, which is crucial in the search for new stressor-specific biomarkers of coastal deterioration. For this purpose, the different tissues (leaf, rhizome, and root) of P. oceanica seagrass from several marine sampling areas were analyzed through target, suspected, and non-target screenings. This paper brings a fast and tissues-specific extraction, as well as a detection method of phenolic compounds applying for the first time the potential of HRMS (Exactive Orbitrap) in P. oceanica samples. As a result, 42 phenolic compounds were satisfactorily detected, of which, to our knowledge, 24 were not previously reported in P. oceanica, such as naringenin, Naringenin chalcone and pinocembrin, among others. Information here reported could be used for the evaluation of new stressor-specific biomarkers of coastal deterioration in the Mediterranean waters. Furthermore, the followed extraction and analytical method could be considered as a reference protocol in other studies on marine seagrasses due to the exhaustive search and satisfactory results.
Identification and Characterization of Chalcone Isomerase Genes Involved in Flavonoid Production in Dracaena cambodiana.[Pubmed:33719287]
Front Plant Sci. 2021 Feb 25;12:616396.
Dragon's blood is a traditional medicine in which flavonoids are the main bioactive compounds; however, the underlying formation mechanism of dragon's blood remains largely poorly understood. Chalcone isomerase (CHI) is the key enzyme in the flavonoid biosynthesis pathway. However, CHI family genes are not well understood in Dracaena cambodiana Pierre ex Gagnep, an important source plant of dragon's blood. In this study, 11 CHI family genes were identified from D. cambodiana, and they were classified into three types. Evolutionary and transcriptional profiling analysis revealed that DcCHI1 and DcCHI4 might be involved in flavonoid production. Both DcCHI1 and DcCHI4 displayed low expression levels in stem under normal growth conditions and were induced by methyl jasmonate (MeJA), 6-benzyl aminopurine (6-BA, synthetic cytokinin), ultraviolet-B (UV-B), and wounding. The recombinant proteins DcCHI1 and DcCHI4 were expressed in Escherichia coli and purified by His-Bind resin chromatography. Enzyme activity assay indicated that DcCHI1 catalyzed the formation of naringenin from Naringenin chalcone, while DcCHI4 lacked this catalytic activity. Overexpression of DcCHI1 or DcCHI4 enhanced the flavonoid production in D. cambodiana and tobacco. These findings implied that DcCHI1 and DcCHI4 play important roles in flavonoid production. Thus, our study will not only contribute to better understand the function and expression regulation of CHI family genes involved in flavonoid production in D. cambodiana but also lay the foundation for developing the effective inducer of dragon's blood.
Functional characterization of two chalcone isomerase (CHI) revealing their responsibility for anthocyanins accumulation in mulberry.[Pubmed:33578286]
Plant Physiol Biochem. 2021 Apr;161:65-73.
Mulberry (Morus sp., Moraceae) is an important economic crop plant and mulberry fruits are rich in anthocyanidins. Chalcone isomerase (CHI) catalyzes the conversion of chalcones to flavanones providing precursors for biosynthesis of anthocyanidins. In this study, bona fide CHIs were cloned and characterized from different Morus species with differently colored fruits (Morus multicaulis, Mm and Morus alba variety LvShenZi, LSZ). Enzymatic assay of MmCHI1 and MmCHI2 showed that they can utilize Naringenin chalcone as substrate. The catalytic efficiency of MmCHI2 and LSZCHI2 are approximately 200 and 120-fold greater than that of MmCHI1 respectively. Phylogenetic analysis showed the two mulberry CHIs belonged to different sub-clade of Type I CHI1 named type IA (CHI2) and type IB (CHI1). Type IB CHIs are mulberry specific. MmCHI1 and MmCHI2 had similar expression profiles and showed preferred expression in fruits. In addition, both mulberry CHI1 and CHI2 played roles in the response to excess zinc stress and sclerotiniose pathogen infection. Both MmCHI1 and MmCHI2 expression levels showed positive close relationship with anthocyanins content during fruit ripening process. The co-expression of MmCHI1 and MmCHI2 was observed during fruit ripening process and in transgenic mulberry. VIGS (virus induced gene silence) targeting on MmCHI1 and MmCHI2 showed significant down-regulation of MmCHI2 instead of MmCHI1 would result in significant (about 50%) decrease in anthocyanins content. MmCHI2 is the dominant CHI for anthocyanins accumulation in mulberry. The results presented in this work provided insight on bona fide CHIs in mulberry and reveal their roles in anthocyanins accumulation.
Discovery of a previously unknown biosynthetic capacity of naringenin chalcone synthase by heterologous expression of a tomato gene cluster in yeast.[Pubmed:33127687]
Sci Adv. 2020 Oct 30;6(44). pii: 6/44/eabd1143.
Chalcone synthase (CHS) canonically catalyzes carbon-carbon bond formation through iterative decarboxylative Claisen condensation. Here, we characterize a previously unidentified biosynthetic capability of SlCHS to catalyze nitrogen-carbon bond formation, leading to the production of a hydroxycinnamic acid amide (HCAA) compound. By expressing a putative tomato (Solanum lycopersicum) gene cluster in yeast (Saccharomyces cerevisiae), we elucidate the activity of a pathway consisting of a carboxyl methyltransferase (SlMT2), which methylates the yeast primary metabolite 3-hydroxyanthranilic acid (3-HAA) to form a methyl ester, and a SlCHS, which catalyzes the condensation of 3-HAA methyl ester and p-coumaroyl-coenzyme A (CoA) through formation of an amide bond. We demonstrate that this aminoacylation activity could be a common secondary activity in plant CHSs by validating the activity in vitro with variants from S. lycopersicum and Arabidopsis thaliana Our work demonstrates yeast as a platform for characterizing putative plant gene clusters with the potential for compound structure and enzymatic activity discovery.
Analysis of secondary metabolites induced by yellowing process for understanding rice yellowing mechanism.[Pubmed:33097330]
Food Chem. 2021 Apr 16;342:128204.
The current study applied wide-targeted metabolomics based approach using LC-ESI-MS/MS to characterize the secondary metabolic difference between yellowed and normal rice. The results indicated that the biosynthesis of secondary metabolites including flavonoids, flavonols and phenolic acids was significantly enhanced during the rice yellowing process, which appears to be highly managed by phenylpropanoid metabolism and flavonoid biosynthetic pathways. Furthermore, rice yellowing led to an increased color parameter b* value, and a number of increased secondary metabolites in the yellowed rice such as homoeriodictyol, Naringenin chalcone, 4,2',4',6'-tetrahydroxychalcone contributed to the yellow color. These may have application as potential biomarkers for characterizing rice yellowing.
Combined Transcriptome and Metabolome analysis of Pitaya fruit unveiled the mechanisms underlying Peel and pulp color formation.[Pubmed:33092530]
BMC Genomics. 2020 Oct 22;21(1):734.
BACKGROUND: Elucidating the candidate genes and key metabolites responsible for pulp and peel coloration is essential for breeding pitaya fruit with new and improved appeal and high nutritional value. Here, we used transcriptome (RNA-Seq) and metabolome analysis (UPLC-MS/MS) to identify structural and regulatory genes and key metabolites associated with peel and pulp colors in three pitaya fruit types belonging to two different Hylocereus species. RESULT: Our combined transcriptome and metabolome analyses suggest that the main strategy for obtaining red color is to increase tyrosine content for downstream steps in the betalain pathway. The upregulation of CYP76ADs is proposed as the color-breaking step leading to red or colorless pulp under the regulation by WRKY44 transcription factor. Supported by the differential accumulation of anthocyanin metabolites in red pulped pitaya fruit, our results showed the regulation of anthocyanin biosynthesis pathway in addition to betalain biosynthesis. However, no color-breaking step for the development of anthocyanins in red pulp was observed and no biosynthesis of anthocyanins in white pulp was found. Together, we propose that red pitaya pulp color is under the strict regulation of CYP76ADs by WRKYs and the anthocyanin coexistence with betalains is unneglectable. We ruled out the possibility of yellow peel color formation due to anthocyanins because of no differential regulation of chalcone synthase genes between yellow and green and no detection of Naringenin chalcone in the metabolome. Similarly, the no differential regulation of key genes in the carotenoid pathway controlling yellow pigments proposed that the carotenoid pathway is not involved in yellow peel color formation. CONCLUSIONS: Together, our results propose several candidate genes and metabolites controlling a single horticultural attribute i.e. color formation for further functional characterization. This study presents useful genomic resources and information for breeding pitaya fruit with commercially attractive peel and pulp colors. These findings will greatly complement the existing knowledge on the biosynthesis of natural pigments for their applications in food and health industry.
Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus.[Pubmed:33089636]
Plant Biotechnol J. 2021 Apr;19(4):671-688.
Flavanones and flavones are excellent source of bioactive compounds but the molecular basis of their highly efficient production remains elusive. Chalcone isomerase (CHI) family proteins play essential roles in flavonoid biosynthesis but little are known about the transcription factors controlling their gene expression. Here, we identified a type IV CHI (designated as CitCHIL1) from citrus which enhances the accumulation of citrus flavanones and flavones (CFLs). CitCHIL1 participates in a CFL biosynthetic metabolon and assists the cyclization of Naringenin chalcone to (2S)-naringenin, which leads to the efficient influx of substrates to chalcone synthase (CHS) and improves the catalytic efficiency of CHS. Overexpressing CitCHIL1 in Citrus and Arabidopsis significantly increased flavonoid content and RNA interference-induced silencing of CitCHIL1 in citrus led to a 43% reduction in CFL content. Three AP2/ERF transcription factors were identified as positive regulators of the CitCHIL1 expression. Of these, two dehydration-responsive element binding (DREB) proteins, CitERF32 and CitERF33, activated the transcription by directly binding to the CGCCGC motif in the promoter, while CitRAV1 (RAV: related to ABI3/VP1) formed a transcription complex with CitERF33 that strongly enhanced the activation efficiency and flavonoid accumulation. These results not only illustrate the specific function that CitCHIL1 executes in CFL biosynthesis but also reveal a new DREB-RAV transcriptional complex regulating flavonoid production.
Homology modeling and molecular dynamics based insights into Chalcone synthase and Chalcone isomerase in Phyllanthus emblica L.[Pubmed:32832333]
3 Biotech. 2020 Aug;10(8):373.
Chalcone synthase (CHS) and chalcone isomerase (CHI) plays a major role in the biosynthesis of flavonoid in plants. In this study, we made extensive bioinformatics analysis to gain functional and structural insight into PeCHS and PeCHI proteins. The phylogenetic distribution of PeCHS and PeCHI genes encoding proteins demonstrated the close evolutionary relationship with different CHS and CHI proteins of other dicot plants. MicroRNA target analysis showed miR169n and 3p miR5053 targeting PeCHS gene while miR169c-3p and miR4248 are targeting PeCHI gene, respectively. Three-dimensional structural models of PeCHS and PeCHI proteins were elucidated by homology modeling with Ramachandran plots showing the excellent geometry of the proteins structure. Molecular docking revealed that cinnamoyl-coa and Naringenin chalcone substrates are strongly bound to PeCHS and PeCHI proteins, respectively. Finally, molecular dynamics (MD) simulation for 30 ns, further yielded stability checks of ligands in the binding pocket and behavior of protein complexes. Thus MD simulation and interaction fraction analysis showed the stable conformation of PeCHS and PeCHI proteins with their respective substrates during theee simulation. Our study provides first-hand structural prospective of PeCHS and PeCHI proteins towards understanding the mechanism of flavonoid biosynthetic pathway in P. emblica.
Synthesis of natural 3'-Prenylchalconaringenin and biological evaluation of ameliorating non-alcoholic fatty liver disease and metabolic syndrome.[Pubmed:32791402]
Eur J Med Chem. 2020 Nov 1;205:112649.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and important risk factor for cardiac diseases, diabetes and extrahepatic cancers. Natural 3'-geranylchalconaringenin (GC) and desmethylxanthohumol (DX) from hop were synthesized using a regio-selective iodination and the Suzuki coupling reaction as key steps. GC and DX, along with their aglycone Naringenin chalcone (NC) were investigated their decreasing the accumulation of cellular lipids. GC reduced lipid content and activated the AMP-activated protein kinase (AMPK) pathway in HepG2 and 3T3-L1 cells. In addition, GC had an obvious therapeutic effect on alleviating NAFLD and metabolic syndrome by activating the AMPK pathway in vivo. In conclusion, GC may be potentially used as a candidate drug and functional food for treating NAFLD and metabolic syndrome.
Two Chalcone Synthase Isozymes Participate Redundantly in UV-Induced Sakuranetin Synthesis in Rice.[Pubmed:32471084]
Int J Mol Sci. 2020 May 27;21(11). pii: ijms21113777.
: Chalcone synthase (CHS) is a key enzyme in the flavonoid pathway, participating in the production of phenolic phytoalexins. The rice genome contains 31 CHS family genes (OsCHSs). The molecular characterization of OsCHSs suggests that OsCHS8 and OsCHS24 belong in the bona fide CHSs, while the other members are categorized in the non-CHS group of type III polyketide synthases (PKSs). Biochemical analyses of recombinant OsCHSs also showed that OsCHS24 and OsCHS8 catalyze the formation of Naringenin chalcone from p-coumaroyl-CoA and malonyl-CoA, while the other OsCHSs had no detectable CHS activity. OsCHS24 is kinetically more efficient than OsCHS8. Of the OsCHSs, OsCHS24 also showed the highest expression levels in different tissues and developmental stages, suggesting that it is the major CHS isoform in rice. In oschs24 mutant leaves, sakuranetin content decreased to 64.6% and 80.2% of those in wild-type leaves at 2 and 4 days after UV irradiation, respectively, even though OsCHS24 expression was mostly suppressed. Instead, the OsCHS8 expression was markedly increased in the oschs24 mutant under UV stress conditions compared to that in the wild-type, which likely supports the UV-induced production of sakuranetin in oschs24. These results suggest that OsCHS24 acts as the main CHS isozyme and OsCHS8 redundantly contributes to the UV-induced production of sakuranetin in rice leaves.
Metabolic Flux Analysis of Catechin Biosynthesis Pathways Using Nanosensor.[Pubmed:32244268]
Antioxidants (Basel). 2020 Mar 31;9(4). pii: antiox9040288.
(+)-Catechin is an important antioxidant of green tea (Camelia sinensis (L.) O. Kuntze). Catechin is known for its positive role in anticancerous activity, extracellular matrix degradation, cell death regulation, diabetes, and other related disorders. As a result of enormous interest in and great demand for catechin, its biosynthesis using metabolic engineering has become the subject of concentrated research with the aim of enhancing (+)-catechin production. Metabolic flux is an essential concept in the practice of metabolic engineering as it helps in the identification of the regulatory element of a biosynthetic pathway. In the present study, an attempt was made to analyze the metabolic flux of the (+)-catechin biosynthesis pathway in order to decipher the regulatory element of this pathway. Firstly, a genetically encoded fluorescence resonance energy transfer (FRET)-based nanosensor (FLIP-Cat, fluorescence indicator protein for (+)-catechin) was developed for real-time monitoring of (+)-catechin flux. In vitro characterization of the purified protein of the nanosensor showed that the nanosensor was pH stable and (+)-catechin specific. Its calculated Kd was 139 microM. The nanosensor also performed real-time monitoring of (+)-catechin in bacterial cells. In the second step of this study, an entire (+)-catechin biosynthesis pathway was constructed and expressed in E. coli in two sets of plasmid constructs: pET26b-PT7-rbs-PAL-PT7-rbs-4CL-PT7-rbs-CHS-PT7-rbs-CHI and pET26b-T7-rbs-F3H-PT7-rbs- DFR-PT7-rbs-LCR. The E. coli harboring the FLIP-Cat was transformed with these plasmid constructs. The metabolic flux analysis of (+)-catechin was carried out using the FLIP-Cat. The FLIP-Cat successfully monitored the flux of catechin after adding tyrosine, 4-coumaric acid, 4-coumaroyl CoA, Naringenin chalcone, naringenin, dihydroquercetin, and leucocyanidin, individually, with the bacterial cells expressing the nanosensor as well as the genes of the (+)-catechin biosynthesis pathway. Dihydroflavonol reductase (DFR) was identified as the main regulatory element of the (+)-catechin biosynthesis pathway. Information about this regulatory element of the (+)-catechin biosynthesis pathway can be used for manipulating the (+)-catechin biosynthesis pathway using a metabolic engineering approach to enhance production of (+)-catechin.


