Grindelic acidCAS# 1438-57-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1438-57-9 | SDF | Download SDF |
| PubChem ID | 20054931 | Appearance | White powder |
| Formula | C20H32O3 | M.Wt | 320.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
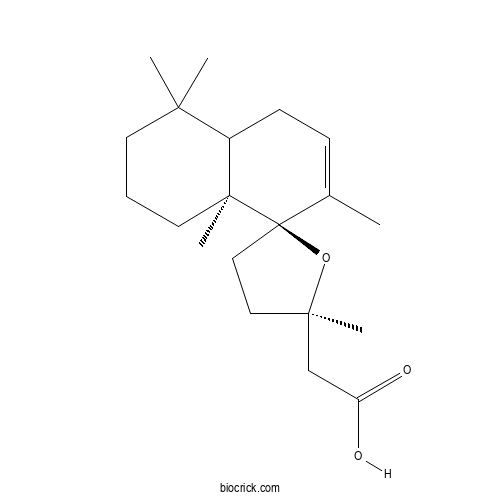
| Chemical Name | 2-[(2'R,8R,8aS)-2',4,4,7,8a-pentamethylspiro[2,3,4a,5-tetrahydro-1H-naphthalene-8,5'-oxolane]-2'-yl]acetic acid | ||
| SMILES | CC1=CCC2C(CCCC2(C13CCC(O3)(C)CC(=O)O)C)(C)C | ||
| Standard InChIKey | XLWWERNKTLITEF-FOUFQHLJSA-N | ||
| Standard InChI | InChI=1S/C20H32O3/c1-14-7-8-15-17(2,3)9-6-10-19(15,5)20(14)12-11-18(4,23-20)13-16(21)22/h7,15H,6,8-13H2,1-5H3,(H,21,22)/t15?,18-,19+,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Grindelic acid Dilution Calculator

Grindelic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1201 mL | 15.6006 mL | 31.2012 mL | 62.4025 mL | 78.0031 mL |
| 5 mM | 0.624 mL | 3.1201 mL | 6.2402 mL | 12.4805 mL | 15.6006 mL |
| 10 mM | 0.312 mL | 1.5601 mL | 3.1201 mL | 6.2402 mL | 7.8003 mL |
| 50 mM | 0.0624 mL | 0.312 mL | 0.624 mL | 1.248 mL | 1.5601 mL |
| 100 mM | 0.0312 mL | 0.156 mL | 0.312 mL | 0.624 mL | 0.78 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fukinolic acid
Catalog No.:BCN0323
CAS No.:50982-40-6
- trans-Ferulic acid
Catalog No.:BCN0322
CAS No.:537-98-4
- Euphorbol
Catalog No.:BCN0321
CAS No.:566-14-3
- Ethyl trans-caffeate
Catalog No.:BCN0320
CAS No.:66648-50-8
- (+/-)-Eriodictyol
Catalog No.:BCN0319
CAS No.:4049-38-1
- Echinatine N-oxide
Catalog No.:BCN0318
CAS No.:20267-93-0
- Diosmetin 7-glucuronide
Catalog No.:BCN0317
CAS No.:35110-20-4
- 2',6'-Dihydroxy 4',4-dimethoxychalcone
Catalog No.:BCN0316
CAS No.:94441-99-3
- Dihydroavenanthramide D
Catalog No.:BCN0315
CAS No.:697235-49-7
- 23-epi-26-Deoxyactein
Catalog No.:BCN0314
CAS No.:501938-01-8
- 5,6-Dehydro 7,8-dihydrokavain
Catalog No.:BCN0313
CAS No.:3155-51-9
- Cycloartenol
Catalog No.:BCN0312
CAS No.:469-38-5
- (Rac)-Hesperetin
Catalog No.:BCN0325
CAS No.:69097-99-0
- 3'-Hydroxyflavone
Catalog No.:BCN0326
CAS No.:70460-18-3
- Hyperforin (stable Dicyclohexylammonium salt)
Catalog No.:BCN0327
CAS No.:238074-03-8
- Isomenthone
Catalog No.:BCN0328
CAS No.:491-07-6
- Isoxanthohumol
Catalog No.:BCN0329
CAS No.:521-48-2
- Lactucin
Catalog No.:BCN0330
CAS No.:1891-29-8
- Lavandulol
Catalog No.:BCN0331
CAS No.:58461-27-1
- Lavandulyl acetate
Catalog No.:BCN0332
CAS No.:25905-14-0
- (+)-Lupanine hydrochloride
Catalog No.:BCN0333
CAS No.:1025-39-4
- 6-Methoxytricin
Catalog No.:BCN0334
CAS No.:76015-42-4
- 16-O-Methylcafestol
Catalog No.:BCN0335
CAS No.:108214-28-4
- Naringenin chalcone
Catalog No.:BCN0336
CAS No.:25515-46-2
Grindelia squarrosa Extract and Grindelic Acid Modulate Pro-inflammatory Functions of Respiratory Epithelium and Human Macrophages.[Pubmed:33536899]
Front Pharmacol. 2021 Jan 18;11:534111.
Aim of the study: Both nasal and bronchial epithelial cells have evolved sophisticated mechanisms involved in cellular response to bacterial infection. Recognition of pathogens by TLR receptors activate the NF-kappaB transcription factor, and lead to production of wide spectrum of cytokines (TNF-alpha, IL-1beta, IL-6 and IL-8). Released by epithelium proinflammatory cytokines intensify migration of macrophages to damaged tissues and modulate their pro-inflammatory functions. Based on traditional use of G. squarrosa aerial parts we hypothesized that successful treatment of cold-related diseases may arise from modulation of the pro-inflammatory functions of respiratory epithelium and human monocytes/macrophages. The biological activity of G. squarrosa extract and Grindelic acid were compared with clarithromycin and budesonide used as positive controls. Methods: The expression of surface receptors (TLR-4, IL-10) and expression of adhesive molecules (ICAM-1, VCAM-1, E-selectin) was analyzed with flow cytometry. The macrophage attachment to the epithelial cells was assessed fluorimetrically. The p65 NF-kappaB concentration and cytokine production was measured spectrophotometrically using enzyme-linked immunosorbent assay. Antibacterial activity was examined by the standard disc-diffusion method and serial dilution method according to CLSI guidelines. Results: G. squarrosa extract and Grindelic acid had no antimicrobial effect. However, we noticed significant modulation of pro-inflammatory functions of LPS-stimulated nasal and bronchial epithelium. G. squarrosa extract treatment resulted in decrease of TLR-4 expression and p65 NF-kappaB concentration and inhibition of cytokines synthesis (IL-8, TNF-alpha, IL-1beta and IL-6) in both cellular models. Additionally, G. squarrosa extract slightly modulated ICAM-1 expression affecting on attachment of macrophages to epithelium. Only G. squarrosa extract was able to stimulate the anti-inflammatory functions of macrophages by inducing TGF-beta release and IL-10 receptor surface expression. Grindelic acid, identified as a dominant compound in the plant extract, modulated pro-inflammatory functions of epithelium and macrophages slightly. Conclusion: The obtained results support traditional use of Grindelia squarrosa preparations for a treatment cold-associated diseases symptoms. In our opinion, the observed biological effect of extract may be a consequence of synergistic effect of all compounds present in the extract.
Inula helenium and Grindelia squarrosa as a source of compounds with anti-inflammatory activity in human neutrophils and cultured human respiratory epithelium.[Pubmed:31644941]
J Ethnopharmacol. 2020 Mar 1;249:112311.
ETHNOPHARMACOLOGICAL RELEVANCE: During the epidemic season, over 90% of acute wheezing disease is associated with bronchial inflammation. Both neutrophil- and eosinophil-mediated inflammation have been involved in the pathophysiology of acute bronchitis, but neutrophil cell recruitment has been shown to be dominant. The ongoing inflammation increases the chemotaxis of neutrophils to inflamed site providing to their overaccumulation. The pharmacological reduction of neutrophil migration can be limited by suppression of major chemo-attractants and cytokines (IL-8, IL-1beta and TNF-alpha) release and downregulation of adhesive molecules. AIM OF THE STUDY: During a screening of plants traditionally used in respiratory tracts diseases (e.g. cough, rhinitis, bronchitis, throat infection, fever, influenza) in Europe, we have selected roots of Inula helenium and aerial parts of Grindelia squarrosa as a potential source of compounds limiting neutrophil migration. MATERIALS AND METHODS: The effect on IL-8, IL-1beta and TNF-alpha release by neutrophils and respiratory epithelium cell line (A549) was measured by enzyme-linked immunosorbent assay (ELISA). The surface expression of adhesive molecules was analyzed with flow cytometry, and the neutrophil attachment to the epithelial cells was assessed fluorimetrically. RESULTS: We confirmed the ability of selected extracts and compounds to suppress neutrophil binding to the epithelium surface via downregulation of beta2 integrin. Alantolactone and Grindelic acid have shown significant suppression of IL-8, TNF-alpha and IL-1beta release comparable with budesonide, used as a positive control. CONCLUSIONS: The present study demonstrated that Inula helenium and Grindelia squarrosa, which have been traditionally used in Europe as medicinal plants, are a valuable source of active compounds with anti-inflammatory activity. Our observations justify the traditional use of I. helenium and G. squarrosa for a treatment of inflammation-based diseases in respiratory tract.
Exploring diterpene metabolism in non-model species: transcriptome-enabled discovery and functional characterization of labda-7,13E-dienyl diphosphate synthase from Grindelia robusta.[Pubmed:26119826]
Plant J. 2015 Sep;83(5):783-93.
Grindelia robusta or gumweed, is a medicinal herb of the sunflower family that forms a diverse suite of diterpenoid natural products. Its major constituents, Grindelic acid and related grindelane diterpenoids accumulate in a resinous exudate covering the plants' surfaces, most prominently the unopened composite flower. Recent studies demonstrated potential pharmaceutical applications for Grindelic acid and its synthetic derivatives. Mining of the previously published transcriptome of G. robusta flower tissue identified two additional diterpene synthases (diTPSs). We report the in vitro and in vivo functional characterization of an ent-kaurene synthase of general metabolism (GrTPS4) and a class II diTPS (GrTPS2) of specialized metabolism that converts geranylgeranyl diphosphate (GGPP) into labda-7,13E-dienyl diphosphate as verified by nuclear magnetic resonance (NMR) analysis. Tissue-specific transcript abundance of GrTPS2 in leaves and flowers accompanied by the presence of an endocyclic 7,13 double bond in labda-7,13E-dienyl diphosphate suggest that GrTPS2 catalyzes the first committed reaction in the biosynthesis of Grindelic acid and related grindelane metabolites. With the formation of labda-7,13E-dienyl diphosphate, GrTPS2 adds an additional function to the portfolio of monofunctional class II diTPSs, which catalytically most closely resembles the bifunctional labda-7,13E-dien-15-ol synthase of the lycopod Selaginella moellendorffii. Together with a recently identified functional diTPS pair of G. robusta producing manoyl oxide, GrTPS2 lays the biosynthetic foundation of the diverse array of labdane-related diterpenoids in the genus Grindelia. Knowledge of these natural diterpenoid metabolic pathways paves the way for developing biotechnology approaches toward producing Grindelic acid and related bioproducts.
Derivatives of grindelic acid: from a non-active natural diterpene to synthetic antitumor derivatives.[Pubmed:23831507]
Eur J Med Chem. 2013 Sep;67:28-38.
Using several reactions that include homologations and asymmetric epoxidations as well as Ugi and Huisgen couplings, we generated a small focused library of new derivatives from the labdane-type diterpene Grindelic acid. These compounds were evaluated as cytotoxic agents against a panel of five human solid tumor cell lines (HBL-100, HeLa, SW1573, T-47D, and WiDr). The presence of the diamide functionalizations enhanced the cytotoxic effect. N-Benzyl-N-(1-(benzylamino)-2-methyl-1-oxopropan-2-yl)grindelicamide, proved to be the most active product in all cell lines tested, with values of 0.95 (+/-0.38) muM against HBL-100 cells.
Stereo- and regioselective hydroxylation of grindelic acid derivatives by Aspergillus niger.[Pubmed:16010831]
Nat Prod Res. 2005 Sep;19(6):625-31.
Stereo- and regioselective hydroxylation of grindelane derivatives on position 3beta was catalyzed by cultures of Aspergillus niger. Grindelic acid (1), methyl grindelate (2), 15-hydroxy-7(8)-en-9alpha,13(S)-oxide-ent-labdane (3) and 7alpha,8alpha-epoxymethylgrindelate (4) were bioconverted into the corresponding 3beta-hydroxy derivatives as the only biotransformation products. 13(S),15-dihydroxy-8(9)-en-ent-labdane (5) remained unreacted under the same conditions. The conformational and electronic studies of the substrates are discussed.
Manoyl oxide alpha-arabinopyranoside and grindelic acid diterpenoids from Grindelia integrifolia.[Pubmed:11678671]
J Nat Prod. 2001 Oct;64(10):1365-7.
Two new manoyl oxide-alpha-arabinopyranoside diterpenoids, 15-hydroxy-13-epi-manoyl oxide-14-O-alpha-L-arabinopyranoside (tarapacol-14-O-alpha-L-arabinopyranoside) (1) and 15-acetoxy-13-epi-manoyl oxide-14-O-alpha-L-arabinopyranoside (tarapacol-15-acetate-14-O-alpha-L-arabinopyranoside) (2), as well as a new Grindelic acid derivative, 19-hydroxyGrindelic acid (3), together with five known diterpenoids (tarapacol, tarapacanol A, Grindelic acid, methyl grindeloate, 3beta-hydroxyGrindelic acid, 4) were isolated from the aerial parts of Grindelia integrifolia. The structures of 1-3 were elucidated by spectral data analysis.
Phytotoxic compounds from Xanthocephalum gymnospermoides var. eradiatum.[Pubmed:11199131]
Planta Med. 2000 Dec;66(8):734-9.
Investigation of the aerial parts of Xanthocephalum gymnospermoides var eradiatum led to the isolation of two new labdane-type of diterpenes, namely, 8 alpha,13S-epoxylabdane-14S,15-diol (1) and methyl grindelate (2). In addition, Grindelic acid (3), 7 alpha, 8 alpha-epoxyGrindelic acid (4), 7 alpha-hydroxy-8(17)dehydroGrindelic acid (5), 17-hydroxyGrindelic acid (6) and 4,5-epoxy-beta-caryophyllene (7) were obtained. The isolated compounds were characterized by spectral means. The absolute configuration of compound 1 was established by chemical correlation with 8 alpha,13S-epoxy-15-nor-labdan-14-oic acid methyl ester of known absolute stereochemistry and by using the advanced Mosher's ester methodology. The results of the present investigation indicated that the known compound barbatol (8) could be an enantiomer of compound 1. Compounds 1-3 and 7 caused significant inhibition of the radicle growth of seedlings of Amaranthus hypochondriacus.
Diterpenoid acids from Grindelia nana.[Pubmed:10757723]
J Nat Prod. 2000 Mar;63(3):378-80.
Two new norditerpenoid acids of the labdane-type (norGrindelic acids), 4,5-dehydro-6-oxo-18-norGrindelic acid (1) and 4beta-hydroxy-6-oxo-19-norGrindelic acid (2), as well as a new Grindelic acid derivative, 18-hydroxy-6-oxoGrindelic acid (3), were isolated from the aerial parts of Grindelia nana. In addition, the known compounds, 6-oxoGrindelic acid, Grindelic acid, methyl grindeloate, 7alpha,8alpha-epoxyGrindelic acid, and 4alpha-carboxyGrindelic acid were also isolated. The structures of the new compounds were characterized on the basis of spectroscopic analysis.


