LavandulolCAS# 58461-27-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 58461-27-1 | SDF | Download SDF |
| PubChem ID | 94060 | Appearance | Colorless-light yellow oily liquid |
| Formula | C10H18O | M.Wt | 154.25 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol and methanol; insoluble in water | ||
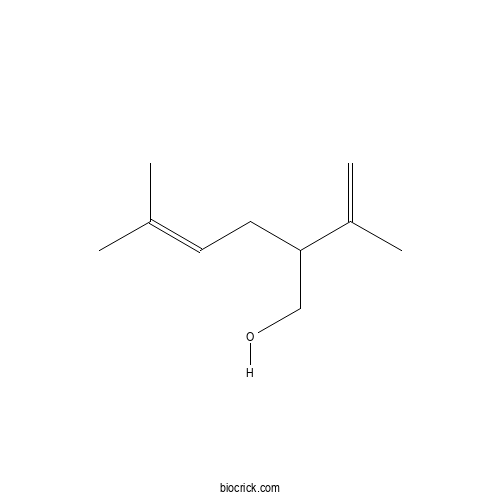
| Chemical Name | 5-methyl-2-prop-1-en-2-ylhex-4-en-1-ol | ||
| SMILES | CC(=CCC(CO)C(=C)C)C | ||
| Standard InChIKey | CZVXBFUKBZRMKR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-8(2)5-6-10(7-11)9(3)4/h5,10-11H,3,6-7H2,1-2,4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lavandulol Dilution Calculator

Lavandulol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.483 mL | 32.4149 mL | 64.8298 mL | 129.6596 mL | 162.0746 mL |
| 5 mM | 1.2966 mL | 6.483 mL | 12.966 mL | 25.9319 mL | 32.4149 mL |
| 10 mM | 0.6483 mL | 3.2415 mL | 6.483 mL | 12.966 mL | 16.2075 mL |
| 50 mM | 0.1297 mL | 0.6483 mL | 1.2966 mL | 2.5932 mL | 3.2415 mL |
| 100 mM | 0.0648 mL | 0.3241 mL | 0.6483 mL | 1.2966 mL | 1.6207 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lactucin
Catalog No.:BCN0330
CAS No.:1891-29-8
- Isoxanthohumol
Catalog No.:BCN0329
CAS No.:521-48-2
- Isomenthone
Catalog No.:BCN0328
CAS No.:491-07-6
- Hyperforin (stable Dicyclohexylammonium salt)
Catalog No.:BCN0327
CAS No.:238074-03-8
- 3'-Hydroxyflavone
Catalog No.:BCN0326
CAS No.:70460-18-3
- (Rac)-Hesperetin
Catalog No.:BCN0325
CAS No.:69097-99-0
- Grindelic acid
Catalog No.:BCN0324
CAS No.:1438-57-9
- Fukinolic acid
Catalog No.:BCN0323
CAS No.:50982-40-6
- trans-Ferulic acid
Catalog No.:BCN0322
CAS No.:537-98-4
- Euphorbol
Catalog No.:BCN0321
CAS No.:566-14-3
- Ethyl trans-caffeate
Catalog No.:BCN0320
CAS No.:66648-50-8
- (+/-)-Eriodictyol
Catalog No.:BCN0319
CAS No.:4049-38-1
- Lavandulyl acetate
Catalog No.:BCN0332
CAS No.:25905-14-0
- (+)-Lupanine hydrochloride
Catalog No.:BCN0333
CAS No.:1025-39-4
- 6-Methoxytricin
Catalog No.:BCN0334
CAS No.:76015-42-4
- 16-O-Methylcafestol
Catalog No.:BCN0335
CAS No.:108214-28-4
- Naringenin chalcone
Catalog No.:BCN0336
CAS No.:25515-46-2
- Pinocembroside
Catalog No.:BCN0337
CAS No.:75829-43-5
- (S)-4',5,7-Trihydroxy-6-prenylflavanone
Catalog No.:BCN0338
CAS No.:68682-01-9
- Sophoraflavanone B
Catalog No.:BCN0339
CAS No.:68682-02-0
- Rebaudioside I
Catalog No.:BCN0340
CAS No.:1220616-34-1
- Rebaudioside O
Catalog No.:BCN0341
CAS No.:1220616-48-7
- Sabinene
Catalog No.:BCN0342
CAS No.:3387-41-5
- (RS)-Sakuranetin
Catalog No.:BCN0343
CAS No.:520-29-6
Development of a Phytochemical-Based Lure for the Dried Bean Beetle Acanthoscelides obtectus Say (Coleoptera: Chrysomelidae).[Pubmed:34370165]
J Chem Ecol. 2021 Aug 9. pii: 10.1007/s10886-021-01305-7.
The dried bean beetle, Acanthoscelides obtectus, is an economically important, worldwide pest of legume crops including dry beans, Phaseolus vulgaris. Assessment of A. obtectus infestation levels in pre-harvest field crops and post-harvest granaries is difficult to achieve because there is no effective monitoring tool for early detection so that interventions can be deployed as needed. Because A. obtectus is a generic pollen and nectar feeder, we adopted an electrophysiological (EAG) screening approach, using the antennae of female A. obtectus to identify physiologically active, volatile phytochemicals, which could then be investigated for their attractiveness to A. obtectus in laboratory behavioral assays and preliminary field tests. Of the 27 compounds tested in EAG screening, 5 compounds, i.e., methyl anthranilate, methyl eugenol, benzyl alcohol, (RS)-Lavandulol, and 2-phenylethanol, elicited stronger EAG responses than the standard (1-phenylethanol). In 4-arm olfactometer bioassays, female A. obtectus preferred the olfactometer arm containing the odor of either methyl anthranilate or benzyl alcohol compared to the solvent control. In preliminary field tests using these 2 compounds as a binary mixture, at least 5 times as many beetles were caught on baited traps compared to non-baited traps. The field data also suggested that benzyl alcohol was primarily responsible for the field activity of the blend. We hypothesize that the attraction of A. obtectus to the combined benzyl alcohol/methyl anthranilate and the single benzyl alcohol baits is connected to the species` nectar- and pollen-feeding behaviour and not to its intraspecific communication. To our knowledge, this is the first evidence that A. obtectus behavior in the field can be modified by the deployment of plant-derived semiochemicals.
Anti-Termitic Activity of Three Plant Extracts, Chlorpyrifos, and a Bioagent Compound (Protecto) against Termite Microcerotermes eugnathus Silvestri (Blattodea: Termitidae) in Egypt.[Pubmed:33158029]
Insects. 2020 Nov 4;11(11). pii: insects11110756.
A trend towards environmentally friendly chemicals for use in termite management has been occurring globally. This study examined three naturally occurring plant extracts from Lavandula latifolia (Spike lavender), Origanum vulgare (Marjorum), and Syzygium aromaticum (Clove) against the termite Microcerotermes eugnathus. Plant extract results were compared to two commercially used termite pesticides, the bio-insecticide, Bacillus thuringiensis var. kurstaki (Protecto 9.4% WP) and Dursban (Chlorpyrifos 48%). Gas chromatography-mass spectrometry (GC-MS) analysis was used to identify the main compounds in the three plant extracts. The main compounds in Lavandula Latifolia were linalool (21.49%), Lavandulol (12.77%), beta-terpinyl acetate (10.49%), and camphor (9.30%). Origanum vulgare extract contained thymol (14.64%), m-cymene (10.63%), linalool (6.75%), and terpinen-4-ol (6.92%) as main compounds. Syzygium aromaticum contained eugenol (99.16%) as the most abundant identified compound. The extract of O. vulgare caused the highest termite death rate, with an LC50 of 770.67 mg/L. Exposure to lavender extract showed a high death rate with an LC50 of 1086.39 mg/L. Clove extract did not show significant insecticidal activity with an LC50 > 2000 mg/L. Significant termiticide effects were found, with LC50 values of 84.09 and 269.98 mg/L for soldiers and workers under the application of Dursban and Protecto, respectively. The LC50 values reported for nymphs were <120, <164.5, and 627.87 mg/L after exposure to Dursban, Protecto, and O. vulgare extract, respectively. The results of the study show that some of the extracts have low toxicity compared to the bioagent and Dursban, and may show promise as natural termiticides, particularly as extracts from O. vulgare.
Biochemical and Histo-Anatomical Responses of Lavandula angustifolia Mill. to Spruce and Beech Bark Extracts Application.[Pubmed:32646016]
Plants (Basel). 2020 Jul 7;9(7). pii: plants9070859.
This paper aims to assess the biological responses of Lavandula angustifolia Mill. to beech and spruce bark crude extract application. Thus, the biological activity of bark extracts was assessed by determining the germination capacity, biomass production, histo-anatomical aspects and photo-assimilatory pigment accumulation, and by quantitative and qualitative volatile compounds analysis. The application of spruce bark extract (500 mg dry bark/100 mL solvent) resulted in a better development of the leaf tissue and an increase in foliar biomass. We observed the stimulating effect of photo-assimilating pigments accumulation, for all experimental variants, compared to the control. Also, the amount of volatile oil was significantly higher in the treated plants with spruce bark extract (500 mg dry bark/100 mL solvent). Some volatile compounds (cyclen, borneol, cryptone, santalen, and caryophyllene oxide beta-farnesene) were identified only in the experimental variants. Also, in the experimental variants, an increase in the quantity of limonene, linalyl acetate and Lavandulol was observed. These preliminary results showed that the beech and spruce bark extracts can have biological activities and influence the production of volatile oil in Lavandula angustifolia, causing significant changes in the phytochemical profile of the essential oil. Thus, forest waste bark extracts could be recommended as natural bioregulators in lavender crops.
Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota.[Pubmed:31500359]
Molecules. 2019 Sep 8;24(18). pii: molecules24183270.
Lavender oil is one of the most valuable aromatherapy oils, its anti-bacterial and anti-fungal activities can be explained by main components such as linalool, linalyl acetate, Lavandulol, geraniol, or eucalyptol. The aim of the study was to assess the anti-microbial effects of two different lavender oils on a mixed microbiota from facial skin. The commercial lavender oil and essential lavender oil from the Crimean Peninsula, whose chemical composition and activity are yet to be published, were used. Both oils were analysed by gas chromatography coupled to mass spectrometry. The composition and properties of studied oils were significantly different. The commercial ETJA lavender oil contained 10% more linalool and linalyl acetate than the Crimean lavender oil. Both oils also had different effects on the mixed facial skin microbiota. The Gram-positive bacilli were more sensitive to ETJA lavender oil, and Gram-negative bacilli were more sensitive to Crimean lavender oil. However, neither of the tested oils inhibited the growth of Gram-positive cocci. The tested lavender oils decreased the cell number of the mixed microbiota from facial skin, but ETJA oil showed higher efficiency, probably because it contains higher concentrations of monoterpenoids and monoterpenes than Crimean lavender oil does.
Characterization of Male-Produced Aggregation Pheromone of the Bean Flower Thrips Megalurothrips sjostedti (Thysanoptera: Thripidae).[Pubmed:30788655]
J Chem Ecol. 2019 Apr;45(4):348-355.
Aggregation of the bean flower thrips, Megalurothrips sjostedti (Trybom) (Thysanoptera: Thripidae), has been observed on cowpea, Vigna unguiculata (L.) Walp. To understand the mechanism underpinning this behavior, we studied the responses of M. sjostedti to headspace volatiles from conspecifics in a four-arm olfactometer. Both male and female M. sjostedti were attracted to male, but not to female odor. Gas chromatography/mass spectrometry (GC/MS) analyses revealed the presence of two distinct compounds in male M. sjostedti headspace, namely (R)-lavandulyl 3-methylbutanoate (major compound) and (R)-Lavandulol (minor compound); by contrast, both compounds were only present in trace amounts in female headspace collections. A behavioral assay using synthetic compounds showed that male M. sjostedti was attracted to both (R)-lavandulyl 3-methylbutanoate and (R)-Lavandulol, while females responded only to (R)-lavandulyl 3-methylbutanoate. This is the first report of a male-produced aggregation pheromone in the genus Megalurothrips. The bean flower thrips is the primary pest of cowpea, which is widely grown in sub-Saharan Africa. The attraction of male and female M. sjostedti to these compounds offers an opportunity to develop ecologically sustainable management methods for M. sjostedti in Africa.
Assessment of chemical and genetic variability in Tanacetum gracile accessions collected from cold desert of Western Himalaya.[Pubmed:29881662]
3 Biotech. 2018 Jun;8(6):284.
Genetic diversity is essential for survival and adaptation of high altitude plants such as those of Tanacetum genus, which are constantly exposed to environmental stress. We collected flowering shoots of ten accessions of Tanacetum gracile Hook.f. & Thomson (Asteraceae) (Tg 1-Tg 10), from different regions of cold desert of Western Himalaya. Chemical profile of the constituents, as inferred from GC-MS, exhibited considerable variability. Percentage yield of essential oil ranged from 0.2 to 0.75% (dry-weight basis) amongst different accessions. Tg 1 and Tg 6 were found to produce high yields of camphor (46%) and Lavandulol (41%), respectively. Alpha-phellendrene, alpha-bisabool, p-cymene and chamazulene were the main oil components in other accessions. Genetic variability among the accessions was studied using RAPD markers as well as by sequencing and analyzing nuclear 18S rDNA, and plastid rbcL and matK loci. The polymorphic information content (PIC) of RAPD markers ranged from 0.18 to 0.5 and the analysis clustered the accessions into two major clades. The present study emphasized the importance of survey, collection, and conservation of naturally existing chemotypes of medicinal and aromatic plants, considering their potential use in aroma and pharmaceutical industry.
Photocatalytic Transfer Hydrogenolysis of Allylic Alcohols on Pd/TiO2 : A Shortcut to (S)-(+)-Lavandulol.[Pubmed:28972300]
Chemistry. 2017 Dec 19;23(71):18025-18032.
We report herein a regio- and stereoselective photocatalytic hydrogenolysis of allylic alcohols to form unsaturated hydrocarbons employing a palladium(II)-loaded titanium oxide; the reaction proceeds at room temperature under light irradiation without stoichiometric generation of salt wastes. Olefin and saturated alcohol moieties tolerated the reaction conditions. Hydrogen atoms were selectively incorporated into less sterically congested carbons of the allylic functionalities. This protocol allowed a short-step synthesis of (S)-(+)-Lavandulol from (R)-(-)-carvone by avoiding otherwise necessary protection/deprotection steps.
Enantioselective comprehensive two-dimensional gas chromatography of lavender essential oil.[Pubmed:27774742]
J Sep Sci. 2016 Dec;39(24):4765-4772.
The enantiomeric composition of several chiral markers in lavender essential oil was studied by flow modulated comprehensive two-dimensional gas chromatography operated in the reverse flow mode and hyphenated to flame ionization and quadrupole mass spectrometric detection. Two capillary column series were used in this study, 2,3-di-O-ethyl-6-O-tert-butyldimethylsilyl-beta-cyclodextrin or 2,3,6-tri-O-methyl-beta-cyclodextrin, as the chiral column in the first dimension and alpha polyethylene glycol column in the second dimension. Combining the chromatographic data obtained on these column series, the enantiomeric and excess ratios for alpha-pinene, beta-pinene, camphor, Lavandulol, borneol, and terpinen-4-ol were determined. This maybe a possible route to assess the authenticity of lavender essential oil.
Pharmacological and Toxicological Studies of Essential Oil of Lavandula stoechas subsp. luisieri.[Pubmed:27124241]
Planta Med. 2016 Sep;82(14):1266-73.
The present study was carried out to evaluate the chemical and pharmacological properties of the essential oil of Lavandula stoechas subsp. luisieri, which is a spontaneous shrub widespread in Alentejo (Portugal). Oxygenated monoterpenes, such as 1,8-cineole, Lavandulol, and necrodane derivatives, are the main components of essential oil. It revealed important antioxidant activity with a high ability to inhibit lipid peroxidation and showed an outstanding effect against a wide spectrum of microorganisms, such as gram-positive and gram-negative bacteria and pathogenic yeasts. The analgesic effect studied in rats was dose dependent, reaching a maximum of 67 % at 60 min with the dose of 200 mg/kg and the anti-inflammatory activity with this dose caused an inhibition in carrageenan-induced rat paw oedema (83 %) that is higher than dexamethasone 1 mg/Kg (69 %). Besides, animals exhibited normal behaviour after essential oil administration, revealing low toxicity. The essential oil of L. luisieri from Alentejo presents important pharmacological properties and low toxicity, and is a promised candidate to be used as a food supplement or in pharmaceutical applications.
Structure and Function of a "Head-to-Middle" Prenyltransferase: Lavandulyl Diphosphate Synthase.[Pubmed:26922900]
Angew Chem Int Ed Engl. 2016 Apr 4;55(15):4721-4.
We report the first X-ray structure of the unique "head-to-middle" monoterpene synthase, lavandulyl diphosphate synthase (LPPS). LPPS catalyzes the condensation of two molecules of dimethylallyl diphosphate (DMAPP) to form lavandulyl diphosphate, a precursor to the fragrance Lavandulol. The structure is similar to that of the bacterial cis-prenyl synthase, undecaprenyl diphosphate synthase (UPPS), and contains an allylic site (S1) in which DMAPP ionizes and a second site (S2) which houses the DMAPP nucleophile. Both S-thiolo-dimethylallyl diphosphate and S-thiolo-isopentenyl diphosphate bind intact to S2, but are cleaved to (thio)diphosphate, in S1. His78 (Asn in UPPS) is essential for catalysis and is proposed to facilitate diphosphate release in S1, while the P1 phosphate in S2 abstracts a proton from the lavandulyl carbocation to form the LPP product. The results are of interest since they provide the first structure and structure-based mechanism of this unusual prenyl synthase.
Cloning and functional characterization of two monoterpene acetyltransferases from glandular trichomes of L. x intermedia.[Pubmed:25998527]
Planta. 2015 Sep;242(3):709-19.
MAIN CONCLUSION: Two alcohol acetyltransferases, LiAAT-3 and LiAAT-4, from L. x intermedia were cloned, expressed in bacteria, and functionally characterized. Two monoterpene acetyltransferase cDNA clones (LiAAT-3 and LiAAT-4) were isolated from L. x intermedia glandular trichomes, expressed in bacteria to produce, and functionally characterize the encoded proteins in vitro. The recombinant LiAAT-3 and LiAAT-4 proteins had molecular weights of ca. 47 and 49 kDa, respectively, as evidenced by SDS-PAGE. The K m (mM) values for the recombinant LiAAT-3 and LiAAT-4 were 1.046 and 0.354 for Lavandulol, 1.31 and 0.279 for geraniol, and 0.87 and 0.113 for nerol, respectively. The V max (pkat/mg) values for LiAAT-3 and LiAAT-4 were 92.13 and 105.1 for Lavandulol, 81.07 and 52.17 for geraniol, and 15.02 and 15.8 for nerol, correspondingly. Catalytic efficiencies (mM(-1) min(-1)) for LiAAT-3 and LiAAT-4 were 0.27 and 0.85 for Lavandulol, 0.19 and 0.54 for geraniol, and 0.052 and 0.4 for nerol, respectively. These kinetic properties are in the range of those reported for other plant acetyltransferases, and indicate that LiAAT-4 has a better catalytic efficiency than LiAAT-3, with Lavandulol serving as the preferred substrate for both enzymes. Transcripts for both genes were abundant in L. angustifolia and L. x intermedia flowers, where monoterpene acetates are produced, and were undetectable (or present in trace quantities) in L. latifolia flowers, which do not accumulate significant amounts of these metabolites.
A new lavandulol-related monoterpene in the sex pheromone of the grey pineapple mealybug, Dysmicoccus neobrevipes.[Pubmed:25618324]
J Chem Ecol. 2015 Feb;41(2):194-201.
The grey pineapple mealybug, Dysmicoccus neobrevipes, is a serious pest that attacks a variety of crops in tropical regions. Recently, it was recorded on an island in southwestern Japan, suggesting that its distribution is expanding. As a measure against this expansion, a monitoring tool is urgently needed. In this study we determined the structure of the sex pheromone of D. neobrevipes in order to develop an efficient lure for monitoring traps. Volatiles collected from virgin adult females were fractionated by high performance liquid chromatography (HPLC) and gas chromatography, and fractions were tested for attractiveness to males in a laboratory bioassay. A single compound was isolated which was as attractive to males as the crude collections, and this was proposed to be the main, if not the only, component of the female-produced sex pheromone. The structure of this was determined to be (E)-2-isopropyl-5-methylhexa-3,5-dienyl acetate by gas chromatography/mass spectrometry and nuclear magnetic resonance analyses. This compound was synthesized through four steps, and the synthetic chemical was as attractive as the natural product in a greenhouse bioassay. The enantiomers of the synthetic acetate were obtained by enantioselective HPLC fractionation of the corresponding alcohols, and the natural pheromone was shown to be the (+)-isomer. The carbon skeleton of this novel compound is related to Lavandulol, a monoterpene with an unusual non-head-to-tail connection of isoprene units that is often found in mealybug pheromones.
Inhibition by TRPA1 agonists of compound action potentials in the frog sciatic nerve.[Pubmed:23537660]
Biochem Biophys Res Commun. 2013 Apr 26;434(1):179-84.
Although TRPV1 and TRPM8 agonists (vanilloid capsaicin and menthol, respectively) at high concentrations inhibit action potential conduction, it remains to be unknown whether TRPA1 agonists have a similar action. The present study examined the actions of TRPA1 agonists, cinnamaldehyde (CA) and allyl isothiocyanate (AITC), which differ in chemical structure from each other, on compound action potentials (CAPs) recorded from the frog sciatic nerve by using the air-gap method. CA and AITC concentration-dependently reduced the peak amplitude of the CAP with the IC50 values of 1.2 and 1.5mM, respectively; these activities were resistant to a non-selective TRP antagonist ruthenium red or a selective TRPA1 antagonist HC-030031. The CA and AITC actions were distinct in property; the latter but not former action was delayed in onset and partially reversible, and CA but not AITC increased thresholds to elicit CAPs. A CAP inhibition was seen by hydroxy-alpha-sanshool (by 60% at 0.05 mM), which activates both TRPA1 and TRPV1 channels, a non-vanilloid TRPV1 agonist piperine (by 20% at 0.07 mM) and tetrahydroLavandulol (where the six-membered ring of menthol is opened; IC50=0.38 mM). It is suggested that TRPA1 agonists as well as TRPV1 and TRPM8 agonists have an ability to inhibit nerve conduction without TRP activation, although their agonists are quite different in chemical structure from each other.


