Stilbostemin BCAS# 162411-67-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 162411-67-8 | SDF | Download SDF |
| PubChem ID | 10376477 | Appearance | Powder |
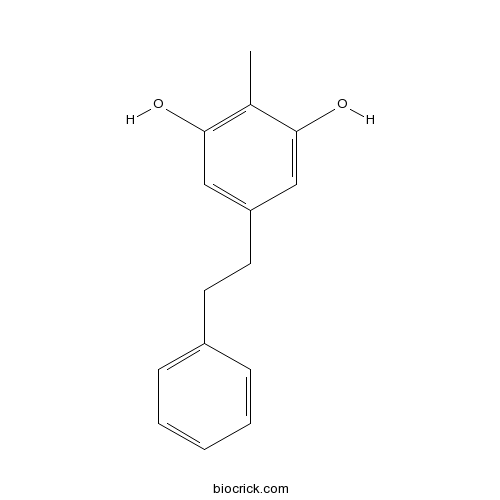
| Formula | C15H16O2 | M.Wt | 228.29 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-methyl-5-(2-phenylethyl)benzene-1,3-diol | ||
| SMILES | CC1=C(C=C(C=C1O)CCC2=CC=CC=C2)O | ||
| Standard InChIKey | ZGLHZPWZOCCDAY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H16O2/c1-11-14(16)9-13(10-15(11)17)8-7-12-5-3-2-4-6-12/h2-6,9-10,16-17H,7-8H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Stilbostemin A, stilbostemin B , stilbostemin D , stilbostemin F , and stilbostemin G show structure-dependent inhibition of leukotriene biosynthesis with IC(50) values ranging from 3.7 to >50 microM. |

Stilbostemin B Dilution Calculator

Stilbostemin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3804 mL | 21.902 mL | 43.8039 mL | 87.6079 mL | 109.5098 mL |
| 5 mM | 0.8761 mL | 4.3804 mL | 8.7608 mL | 17.5216 mL | 21.902 mL |
| 10 mM | 0.438 mL | 2.1902 mL | 4.3804 mL | 8.7608 mL | 10.951 mL |
| 50 mM | 0.0876 mL | 0.438 mL | 0.8761 mL | 1.7522 mL | 2.1902 mL |
| 100 mM | 0.0438 mL | 0.219 mL | 0.438 mL | 0.8761 mL | 1.0951 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GR 103691
Catalog No.:BCC6941
CAS No.:162408-66-4
- 3-Cyclopropylmethoxy-4-difluoromethoxybenzoic acid
Catalog No.:BCC8628
CAS No.:162401-62-9
- Roflumilast
Catalog No.:BCN2182
CAS No.:162401-32-3
- 3-O-Methyl-Estrone
Catalog No.:BCC8640
CAS No.:1624-62-0
- Vibralactone L
Catalog No.:BCN6914
CAS No.:1623786-67-3
- Vibralactone K
Catalog No.:BCN6746
CAS No.:1623786-66-2
- 3-Amino-3-(hydroxymethyl)-1-(4-octylphenyl)-1,4-butanediol
Catalog No.:BCN1541
CAS No.:162361-49-1
- Fingolimod hydrochloride
Catalog No.:BCN2167
CAS No.:162359-56-0
- 2-Amino-2-[2-(4-octylphenyl)ethyl]-1,3-propandiol
Catalog No.:BCN1542
CAS No.:162359-55-9
- Acetamide, N-[1,1-bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]-
Catalog No.:BCN2254
CAS No.:162358-09-0
- Diethyl 2-acetamido-2-(4-octylphenethyl)malonate
Catalog No.:BCN1543
CAS No.:162358-08-9
- 1-(2-iodoethyl)-4-octylbenzene
Catalog No.:BCN2253
CAS No.:162358-07-8
- VR23
Catalog No.:BCC6523
CAS No.:1624602-30-7
- Subelliptenone G
Catalog No.:BCN1720
CAS No.:162473-22-5
- Salirasib
Catalog No.:BCC1918
CAS No.:162520-00-5
- CDP 840 hydrochloride
Catalog No.:BCC7814
CAS No.:162542-90-7
- Fmoc-Dap(Boc)-OH
Catalog No.:BCC3188
CAS No.:162558-25-0
- Broussoflavonol F
Catalog No.:BCN3571
CAS No.:162558-94-3
- 3-Hydroxy-5,7-dimethoxy-3',4'-methylenedioxyflavan
Catalog No.:BCN1540
CAS No.:162602-04-2
- Isolupalbigenin
Catalog No.:BCN6835
CAS No.:162616-70-8
- Eriosemation
Catalog No.:BCN3738
CAS No.:162616-72-0
- Sorokinianin
Catalog No.:BCN6978
CAS No.:162616-73-1
- Temsirolimus
Catalog No.:BCC3678
CAS No.:162635-04-3
- AZD3759
Catalog No.:BCC6475
CAS No.:1626387-80-1
[Chemical constituents from Glechoma longituba].[Pubmed:25204149]
Zhongguo Zhong Yao Za Zhi. 2014 Feb;39(4):695-8.
Fourteen compounds were obtained from Glechoma longituba by the chromatographic methods of silica gel, ODS, Sephadex LH-20 and preparative of HPLC. According to physicochemical properties and spectral data, these compounds were identified as Stilbostemin B (1), trilepisiumic acid (2), 3, 4-dihydroxyphenyl ethanol ketone (3), bergeninmonohydrate (4), oresbiusin A (5), norbergenin (6), stilbostemin D (7), ehretioside B (8), ethyl ferulate (9), E-p-hydroxy-cinnamic acid (10), methyl gallate (11), protocatechuic acid (12), 4'-Hydroxyacetophenone (13), and E-3-2,4-dihydroxyphenyl-2-acrylic acid (14). Among them, compounds 1-10, 13 and 14 were isolated from this plant for the first time.
Neuroprotective bibenzyl glycosides of Stemona tuberosa roots.[Pubmed:16643052]
J Nat Prod. 2006 Apr;69(4):679-81.
Three new bibenzyl glycosides characterized as Stilbostemin B 3'-beta-D-glucopyranoside (1), stilbostemin H 3'-beta-D-glucopyranoside (2), and stilbostemin I 2"-beta-D-glucopyranoside (3) were isolated from the roots of Stemona tuberosa. All three bibenzyl glycosides significantly protected human neuroblastoma SH-SY5Y cells from 6-hydroxydopamine-induced neurotoxicity.
Inhibition of leukotriene biosynthesis by stilbenoids from Stemona species.[Pubmed:15679323]
J Nat Prod. 2005 Jan;68(1):83-5.
Fifteen stilbenoids and two alkaloids from Stemona collinsae, S. tuberosa, and S. peirrei were tested alongside the commercially available stilbenoids resveratrol and pinosylvin for inhibition of leukotriene formation in an ex vivo test system based on activated human neutrophilic granulocytes. The stilbenoids resveratrol (1), pinosylvin (2), dihydropinosylvin (3), stilbostemin A (4), Stilbostemin B (5), stilbostemin D (6), stilbostemin F (7), stilbostemin G (8), stemofuran B (9), stemofuran C (10), stemofuran D (11), stemofuran G (12), stemofuran J (13), stemanthrene A (14), stemanthrene B (15), stemanthrene C (16), and stemanthrene D (17) showed structure-dependent activities with IC(50) values ranging from 3.7 to >50 microM. The alkaloids tuberostemonine (18) and neotuberostemonine (19) were inactive at a concentration of 50 microM.


