CDP 840 hydrochloridePDE4 inhibitor,potent and selective CAS# 162542-90-7 |
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- LY2857785
Catalog No.:BCC8050
CAS No.:1619903-54-6
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- P276-00
Catalog No.:BCC4415
CAS No.:920113-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 162542-90-7 | SDF | Download SDF |
| PubChem ID | 127928 | Appearance | Powder |
| Formula | C25H27NO2 | M.Wt | 373.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
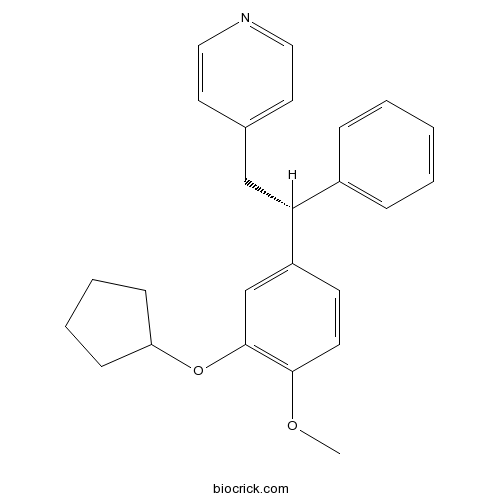
| Chemical Name | 4-[(2R)-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-phenylethyl]pyridine | ||
| SMILES | COC1=C(C=C(C=C1)C(CC2=CC=NC=C2)C3=CC=CC=C3)OC4CCCC4 | ||
| Standard InChIKey | UTUUPXBCDMQYRR-HSZRJFAPSA-N | ||
| Standard InChI | InChI=1S/C25H27NO2/c1-27-24-12-11-21(18-25(24)28-22-9-5-6-10-22)23(20-7-3-2-4-8-20)17-19-13-15-26-16-14-19/h2-4,7-8,11-16,18,22-23H,5-6,9-10,17H2,1H3/t23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective inhibitor of phosphodiesterase (PDE) 4 (IC50 = 12 nM for native PDE4). Competitively inhibits all PDE4 isoenzymes (IC50 values are 4, 9, 9 and 45 nM for PDE4A, PDE4B, PDE4C and PDE4D human recombinant isoforms expressed in yeast). |

CDP 840 hydrochloride Dilution Calculator

CDP 840 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6774 mL | 13.3869 mL | 26.7738 mL | 53.5475 mL | 66.9344 mL |
| 5 mM | 0.5355 mL | 2.6774 mL | 5.3548 mL | 10.7095 mL | 13.3869 mL |
| 10 mM | 0.2677 mL | 1.3387 mL | 2.6774 mL | 5.3548 mL | 6.6934 mL |
| 50 mM | 0.0535 mL | 0.2677 mL | 0.5355 mL | 1.071 mL | 1.3387 mL |
| 100 mM | 0.0268 mL | 0.1339 mL | 0.2677 mL | 0.5355 mL | 0.6693 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CDP 840 is a selective phosphodiesterase type IV (PDE4) inhibitor [1] [2] [3] with an IC50 value of 0.007 µM [4].
PDE4 is most abundantly distributed in inflammatory cells such as monocytes and macrophages [3]. PDE4 is a high-affinity cAMP-selective isozyme. It was found that PDE4 was in almost all cell types in asthma pathogenesis [2].
CD P840 showed a potent inhibition against PDE4 with IC50 values ranging from 2-30 nM to different isoenzymes of PDE4. There are four expressed PDE4 isoenzymes in baculovirus cells, i.e. PDE4A, PDE4B, PDE4C and PDE4D. Except for PDE4C2, CDP 840 did not exhibit isoform selectivity of PDE4. CD P840 exhibited a hill number of about 1.0 against all four PDE4 isoenzymes. For all four PDE4 isoenzymes, CDP 840 acted as a simple competitive inhibitor [5].
In vivo, CDP 840 (30 mg/kg) increased cAMP by 145% in the hippocampus and by 112% in the prefrontal cortex of male Sprague-Dawley rats. CDP 840 at doses of 10 and 30 mg/kg increased the phosphorylation of cAMP response element binding protein (pCREB) in the hippocampus (by 36 and 55%, respectively) and in the prefrontal cortex (by 32 and 60%, respectively). But these doses did not affect the expression of the cAMP response element binding protein (CREB). Repeated treatment with CDP 840 at a dose of 30 mg/kg increased the cell proliferation in rat hippocampus, but these cells were not survival [6].
References:
[1]. T.R. Jones, M. McAuliffe, C.S. McFarlane, et al. Effects of a selective phosphodiesterase IV inhibitor (CDP-840) in a leukotriene-dependent non-human primate model of allergic asthma. Can. J. Physiol. Pharmacol., 1998, 76: 210-217.
[2]. Chun Li, Nathalie Chauret, Laird A. Trimble, et al. Investigation of the in vitro metabolism profile of a phosphodiesterase-IV inhibitor, CDP-840: leading to structural optimization. Drug Metabolism and Disposition, 2001, 29:232–241.
[3]. John E. Souness and Sudha Rao. Proposal for Pharmacologically Distinct Conformers of PDE4 Cyclic AMP Phosphodiesterases. Cell. Signal., 1997, 9(3/4):227-236.
[4]. Christopher Hulme, Gregory B. Poli, Fu-Chih Huang, et al. Quaternary substituted PDE4 inhibitors I: the synthesis and in vitro evaluation of a novel series of oxindoles. Bioorganic & Medicinal Chemistry Letters, 1998, 8:175-178.
[5]. M.J. Perry, J. O'Connell, C. Walker, et al. CDP840: a novel inhibitor of PDE-4. Cell Biochem. Biophys., 1998, 29(1-2):113-32.
[6]. Lan Xiao, James P. O’Callaghan and James M. O’Donnell. Effects of Repeated Treatment with Phosphodiesterase-4 Inhibitors on cAMP Signaling, Hippocampal Cell Proliferation, and Behavior in the Forced-Swim Test. J. Pharmacol. Exp. Ther., 2011, 338(2):641-7.
- Salirasib
Catalog No.:BCC1918
CAS No.:162520-00-5
- Subelliptenone G
Catalog No.:BCN1720
CAS No.:162473-22-5
- VR23
Catalog No.:BCC6523
CAS No.:1624602-30-7
- Stilbostemin B
Catalog No.:BCN4697
CAS No.:162411-67-8
- GR 103691
Catalog No.:BCC6941
CAS No.:162408-66-4
- 3-Cyclopropylmethoxy-4-difluoromethoxybenzoic acid
Catalog No.:BCC8628
CAS No.:162401-62-9
- Roflumilast
Catalog No.:BCN2182
CAS No.:162401-32-3
- 3-O-Methyl-Estrone
Catalog No.:BCC8640
CAS No.:1624-62-0
- Vibralactone L
Catalog No.:BCN6914
CAS No.:1623786-67-3
- Vibralactone K
Catalog No.:BCN6746
CAS No.:1623786-66-2
- 3-Amino-3-(hydroxymethyl)-1-(4-octylphenyl)-1,4-butanediol
Catalog No.:BCN1541
CAS No.:162361-49-1
- Fingolimod hydrochloride
Catalog No.:BCN2167
CAS No.:162359-56-0
- Fmoc-Dap(Boc)-OH
Catalog No.:BCC3188
CAS No.:162558-25-0
- Broussoflavonol F
Catalog No.:BCN3571
CAS No.:162558-94-3
- 3-Hydroxy-5,7-dimethoxy-3',4'-methylenedioxyflavan
Catalog No.:BCN1540
CAS No.:162602-04-2
- Isolupalbigenin
Catalog No.:BCN6835
CAS No.:162616-70-8
- Eriosemation
Catalog No.:BCN3738
CAS No.:162616-72-0
- Sorokinianin
Catalog No.:BCN6978
CAS No.:162616-73-1
- Temsirolimus
Catalog No.:BCC3678
CAS No.:162635-04-3
- AZD3759
Catalog No.:BCC6475
CAS No.:1626387-80-1
- AT 56
Catalog No.:BCC6036
CAS No.:162640-98-4
- HQL 79
Catalog No.:BCC7703
CAS No.:162641-16-9
- 6-Deoxyjacareubin
Catalog No.:BCN6573
CAS No.:16265-56-8
- Kaempferol tetraacetate
Catalog No.:BCN1721
CAS No.:16274-11-6
A comparison of the inhibitory activity of PDE4 inhibitors on leukocyte PDE4 activity in vitro and eosinophil trafficking in vivo.[Pubmed:10372831]
Br J Pharmacol. 1999 Apr;126(8):1863-71.
1. Phosphodiesterase (PDE) 4 inhibitors have been shown to inhibit eosinophil PDE4 activity in vitro and accumulation of eosinophils in experimental airways inflammation. However, direct effects on eosinophil trafficking have not been studied in detail and it is not known if activity in vitro translates into efficacy in vivo. In the present study, we compared the activity of five PDE4 inhibitors in vitro and against trafficking of (111)In-eosinophils in cutaneous inflammation in the guinea-pig. 2. The rank order of potency for inhibition of PDE4 activity in guinea-pig eosinophil, neutrophil and macrophage, and human neutrophil lysates was RP73401 > SB207499 >CDP840 > rolipram > LAS31025. On TNFalpha production by human PBMC, all inhibitors with the exception of rolipram showed potency similar to their effect on neutrophil lysates. 3. In a brain cerebellum binding assay, the rank order of potency at displacing [3H]-rolipram was RP73401 > rolipram > SB207499 > CDP840 > LAS30125. 4. Trafficking of (111)In-eosinophils to skin sites injected with PAF, ZAP or antigen in sensitized sites was inhibited by oral administration of all PDE4 inhibitors. The rank order of potency was RP73401 = rolipram > LAS31025 > SB207499 > CDP840. 5. With the exception was RP73401, which was the most potent compound in all assays, there was no clear relationship between activity of PDE4 inhibitors in vitro and capacity to inhibit eosinophil trafficking in vivo. Thus, we conclude that in vitro activity of PDE4 inhibitors does not predict in vivo efficacy in an experimental model of eosinophil trafficking.
CDP840: a novel inhibitor of PDE-4.[Pubmed:9631241]
Cell Biochem Biophys. 1998;29(1-2):113-32.
We present the in vitro characterization of a novel phosphodiesterase type 4 inhibitor, CDP840 (R-[+]-4-[2- inverted question mark3-cyclopentyloxy-4-methoxyphenyl inverted question mark-2-phenylethyl]pyridine), which has shown efficacy in a phase II allergen challenge study in asthmatics without adverse effects. CDP840 potently inhibits PDE-4 isoenzymes (IC50 2-30 nM) without any effect on PDE-1, 2, 3, 5, and 7 (IC50 > 100 microM). It exhibited no significant selectivity in inhibiting human recombinant isoenzymes PDE-4A, B, C or D and was equally active against the isoenzymes lacking UCR1 (PDE-4B2 and PDE-4D2). In contrast to rolipram, CDP840 acted as a simple competitive inhibitor of all PDE-4 isoenzymes. Studies with rolipram indicated a heterogeneity within all the preparations of PDE-4 isoenzymes, indicative of rolipram inhibiting the catalytic activity of PDE-4 with both a low or high affinity. These observations were confirmed by the use of a PDE-4A variant, PDE-4A330-886, which rolipram inhibited with low affinity (IC50 = 1022 nM). CDP840 in contrast inhibited this PDE-4A variant with similar potency (IC50 = 3.9 nM), which was in good agreement with the Kd of 4.8 nM obtained from [3H]-CDP840 binding studies. Both CDP840 and rolipram inhibited the high-affinity binding of [3H]-rolipram binding to PDE-4A, B, C, and D with similar Kd app (7-19 nM and 3-5 nM, respectively). Thus, the activity of CDP840 at the [3H]-rolipram binding site was in agreement with the inhibitor's activity at the catalytic site. However, rolipram was approximately 100-fold more potent than CDP840 at inhibiting the binding of [3H]-rolipram to mouse brain in vivo. These data clearly demonstrate that CDP840 is a potent selective inhibitor of all PDE-4 isoenzymes. In contrast to rolipram, CDP840 was well-tolerated in humans. This difference, however, cannot at present be attributed to either isoenzyme selectivity or lack of activity in vitro at the high-affinity rolipram binding site (Sr).
The inhibition of antigen-induced eosinophilia and bronchoconstriction by CDP840, a novel stereo-selective inhibitor of phosphodiesterase type 4.[Pubmed:8818342]
Br J Pharmacol. 1996 Jul;118(5):1183-91.
1. The novel tri-aryl ethane CDP840, is a potent and selective inhibitor of cyclic AMP phosphodiesterase type 4 (PDE 4) extracted from tissues or recombinant PDE 4 isoforms expressed in yeast (IC50S: 4-45 nM). CDP840 is stereo-selective since its S enantiomer (CT 1731) is 10-50 times less active against all forms of PDE 4 tested while both enantiomers are inactive (IC50S: > 100 microM) against PDE types 1, 2, 3 and 5. 2. Oral administration of CDP840 caused a dose-dependent reduction of interleukin-5 (IL-5)-induced pleural eosinophilia in rats (ED50 = 0.03 mg kg-1). The eosinophils in pleural exudates from CDP840-treated animals contained higher levels of eosinophil peroxidase (EPO) than cells from control animals, suggesting a stabilizing effect on eosinophil degranulation. CDP840 was approximately equi-active with the steroid dexamethasone in this model and was 10-100 times more potent than the known PDE 4-selective inhibitors rolipram and RP73401. The activity of CDP840 was not influenced by adrenalectomy, beta-sympathomimetics or beta-sympatholytics. 3. Antigen-induced pulmonary eosinophilia in sensitized guinea-pigs was reduced dose-dependently by CDP840 (0.01-1 mg kg-1, i.p.) and intracellular EPO levels were significantly higher. CDP840 was more potent in these activities than CT1731 or rolipram and comparable in potency to RP73401. 4. Rolipram or CDP840 were less active than dexamethasone in preventing neutrophil accumulation, or exudate formation in carrageenan-induced pleurisy in rats and thus do not exhibit general anti-inflammatory activity. 5. In sensitized guinea-pigs, aerosols of the antigen ovalbumin caused a dose-dependent bronchoconstriction demonstrated by an increase in pulmonary inflation pressure. Administration of CDP840 (0.001-1.0 mg kg-1, i.p.), 1 h before antigen challenge, resulted in dose-dependent reduction in response to antigen. This activity was not due to bronchodilatation since higher doses of CDP840 (3 mg kg-1) did not significantly change the bronchoconstrictor response to histamine. Rolipram was approximately 10 times less active than CDP840 in preventing antigen-induced bronchoconstriction. 6. These results confirm the observations that selective PDE 4 inhibitors reduce antigen-induced bronchoconstriction and pulmonary eosinophilic inflammation. CDP840 is more potent than rolipram in inhibiting native or recombinant PDE 4. Unlike the recently described potent PDE 4 inhibitor RP73401, CDP840 is more active than rolipram in the rat IL-5 model following oral administration. The novel series of tri-aryl ethanes, of which CDP840 is the lead compound, could be the basis of an orally active prophylactic treatment for human asthma.


