RoflumilastPDE-4 inhibitor CAS# 162401-32-3 |
- A-867744
Catalog No.:BCC1324
CAS No.:1000279-69-5
- Rocuronium Bromide
Catalog No.:BCC1068
CAS No.:119302-91-9
- Rivastigmine
Catalog No.:BCC1900
CAS No.:123441-03-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 162401-32-3 | SDF | Download SDF |
| PubChem ID | 449193 | Appearance | Powder |
| Formula | C17H14Cl2F2N2O3 | M.Wt | 403.21 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (124.00 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
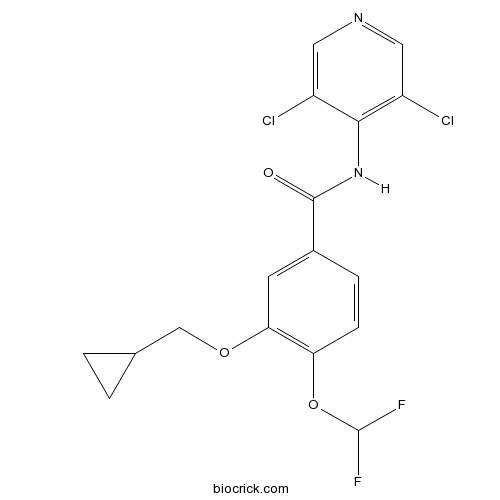
| Chemical Name | 3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide | ||
| SMILES | C1CC1COC2=C(C=CC(=C2)C(=O)NC3=C(C=NC=C3Cl)Cl)OC(F)F | ||
| Standard InChIKey | MNDBXUUTURYVHR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Roflumilast is a phosphodiesterase 4 inhibitor that may improve lung function and reduce the frequency of exacerbations in patients with COPD. 2. Suppression of hematological and immunological markers of inflammation and enhanced apoptosis in animals treated with Roflumilast points to the possibility of a beneficial effect of Roflumilast in allergic inflammation. 3. Roflumilast has anti-inflammatory and immunomodulatory potential,it will be useful in the treatment of chronic inflammatory disorders such as asthma and chronic obstructive pulmonary disease. |
| Targets | cAMP | ROS | IL Receptor | PDE |

Roflumilast Dilution Calculator

Roflumilast Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4801 mL | 12.4005 mL | 24.801 mL | 49.6019 mL | 62.0024 mL |
| 5 mM | 0.496 mL | 2.4801 mL | 4.9602 mL | 9.9204 mL | 12.4005 mL |
| 10 mM | 0.248 mL | 1.24 mL | 2.4801 mL | 4.9602 mL | 6.2002 mL |
| 50 mM | 0.0496 mL | 0.248 mL | 0.496 mL | 0.992 mL | 1.24 mL |
| 100 mM | 0.0248 mL | 0.124 mL | 0.248 mL | 0.496 mL | 0.62 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Roflumilast is an orally active, selective inhibitor of phophodiesterase-4 (PDE-4) with IC50 value of 0.11nM [1].
Roflumilast is developed for the treatment of the inflammatory component of COPD since PDE-4 is found mainly in inflammatory cells. In vitro studies show that roflumilast inhibits the production of inflammatory mediators in a variety of human immune cells, suggesting a role for reducing COPD-related inflammation. Roflumilast has a rapid absorption with a bioavailability of ~ 80% and the half-life is approximately 17h after repeated dosing of 500 mcg OD. Pharmacokinetic studies show no significant interactions with the drugs used by COPD patients. Roflumilast is also found to decrease glucose levels in patients with newly diagnosed type-2 diabetes since diabetes is an important comorbidity associated with COPD [2].
References:
[1] Z Huang, JA Mancini. Phosphodiesterase 4 Inhibitors for the Treatment of Asthma and COPD. Current Medicinal Chemistry, 2006.
[2] Tashkin DP. Roflumilast : the new orally active, selective phophodiesterase-4 inhibitor, for the treatment of COPD. Expert Opin Pharmacother. 2014 Jan;15(1):85-96.
- 3-O-Methyl-Estrone
Catalog No.:BCC8640
CAS No.:1624-62-0
- Vibralactone L
Catalog No.:BCN6914
CAS No.:1623786-67-3
- Vibralactone K
Catalog No.:BCN6746
CAS No.:1623786-66-2
- 3-Amino-3-(hydroxymethyl)-1-(4-octylphenyl)-1,4-butanediol
Catalog No.:BCN1541
CAS No.:162361-49-1
- Fingolimod hydrochloride
Catalog No.:BCN2167
CAS No.:162359-56-0
- 2-Amino-2-[2-(4-octylphenyl)ethyl]-1,3-propandiol
Catalog No.:BCN1542
CAS No.:162359-55-9
- Acetamide, N-[1,1-bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]-
Catalog No.:BCN2254
CAS No.:162358-09-0
- Diethyl 2-acetamido-2-(4-octylphenethyl)malonate
Catalog No.:BCN1543
CAS No.:162358-08-9
- 1-(2-iodoethyl)-4-octylbenzene
Catalog No.:BCN2253
CAS No.:162358-07-8
- Benzeneethanol, 4-octyl
Catalog No.:BCN2255
CAS No.:162358-05-6
- Benzeneethanol, 4-octyl-, 1-acetate
Catalog No.:BCN2252
CAS No.:162358-04-5
- 1-Octanone,1-[4-[2-(acetyloxy)ethyl]phenyl]
Catalog No.:BCN2251
CAS No.:162358-03-4
- 3-Cyclopropylmethoxy-4-difluoromethoxybenzoic acid
Catalog No.:BCC8628
CAS No.:162401-62-9
- GR 103691
Catalog No.:BCC6941
CAS No.:162408-66-4
- Stilbostemin B
Catalog No.:BCN4697
CAS No.:162411-67-8
- VR23
Catalog No.:BCC6523
CAS No.:1624602-30-7
- Subelliptenone G
Catalog No.:BCN1720
CAS No.:162473-22-5
- Salirasib
Catalog No.:BCC1918
CAS No.:162520-00-5
- CDP 840 hydrochloride
Catalog No.:BCC7814
CAS No.:162542-90-7
- Fmoc-Dap(Boc)-OH
Catalog No.:BCC3188
CAS No.:162558-25-0
- Broussoflavonol F
Catalog No.:BCN3571
CAS No.:162558-94-3
- 3-Hydroxy-5,7-dimethoxy-3',4'-methylenedioxyflavan
Catalog No.:BCN1540
CAS No.:162602-04-2
- Isolupalbigenin
Catalog No.:BCN6835
CAS No.:162616-70-8
- Eriosemation
Catalog No.:BCN3738
CAS No.:162616-72-0
Roflumilast-induced Local Vascular Injury Is Associated with a Coordinated Proteome and Microparticle Change in the Systemic Circulation in Pigs.[Pubmed:25311372]
Toxicol Pathol. 2015 Jun;43(4):569-80.
Drug-induced vascular injury (DIVI) is commonly associated with phosphodiesterase (PDE) inhibitors. Despite histological characterization, qualified biomarkers for DIVI detection are lacking. We investigated whether a single administration of Roflumilast (PDE-IV inhibitor) induces vascular damage and identified novel surrogate biomarkers of acute vascular injury. Pigs received postoperative 250, 375, or 500 mug of Roflumilast or placebo/control. After 1.5 hr, coronary reactivity was determined by catheter-based administration of acetylcholine and sodium nitroprusside (SNP) in the coronary sinus. Immunohistochemical analysis of vessel integrity (von Willebrand factor [vWF]) and fibrin(ogen) deposition was performed in the coronary artery and aorta. Peripheral blood was collected for differential proteomics and microparticles analysis. Circulating interleukin (IL)-6 was analyzed. Roflumilast-treated animals displayed higher vasodilation to acetylcholine and SNP versus controls (p < .05). Roflumilast-treated animals showed a dose-dependent (p < .05) decrease in vessel integrity and dose-dependent increase in fibrin deposition forming a continuous layer at Roflumilast-500 mug. Peripheral blood of Roflumilast-500-mug-treated animals showed increased levels of total and endothelial-derived microparticles and exhibited a coordinated change in proteins kininogen-1, endothelin-1, gelsolin, apolipoprotein A-I, and apolipoprotein-J associated with vascular injury (p < .05 vs. controls). IL-6 remained unaltered. Roflumilast-induced vascular injury can be detected by novel markers in peripheral blood. Validation of these surrogate markers in human samples seems required.
Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro.[Pubmed:11259554]
J Pharmacol Exp Ther. 2001 Apr;297(1):267-79.
From a series of benzamide derivatives, Roflumilast (3-cyclo-propylmethoxy-4-difluoromethoxy-N-[3,5-di-chloropyrid-4-yl]-benzamide) was identified as a potent and selective PDE4 inhibitor. It inhibits PDE4 activity from human neutrophils with an IC(50) of 0.8 nM without affecting PDE1 (bovine brain), PDE2 (rat heart), and PDE3 and PDE5 (human platelets) even at 10,000-fold higher concentrations. Roflumilast is almost equipotent to its major metabolite formed in vivo (Roflumilast N-oxide) and piclamilast (RP 73401), however, more than 100-fold more potent than rolipram and Ariflo (cilomilast; SB 207499). The anti-inflammatory and immunomodulatory potential of Roflumilast and the reference compounds was investigated in various human leukocytes using cell-specific responses: neutrophils [N-formyl-methyl-leucyl-phenylalanine (fMLP)-induced formation of LTB(4) and reactive oxygen species (ROS)], eosinophils (fMLP- and C5a-induced ROS formation), monocytes, monocyte-derived macrophages, and dendritic cells (lipopolysaccharide-induced tumor necrosis factor-alpha synthesis), and CD4+ T cells (anti-CD3/anti-CD28 monoclonal antibody-stimulated proliferation, IL-2, IL-4, IL-5, and interferon-gamma release). Independent of the cell type and the response investigated, the corresponding IC values (for half-maximum inhibition) of Roflumilast were within a narrow range (2-21 nM), very similar to Roflumilast N-oxide (3-40 nM) and piclamilast (2-13 nM). In contrast, cilomilast (40-3000 nM) and rolipram (10-600 nM) showed greater differences with the highest potency for neutrophils. Compared with neutrophils and eosinophils, representing the terminal inflammatory effector cells, the relative potency of Roflumilast and its N-oxide for monocytes, CD4+ T cells, and dendritic cells is substantially higher compared with cilomilast and rolipram, probably reflecting an improved immunomodulatory potential. The efficacy of Roflumilast in vitro and in vivo (see accompanying article in this issue) suggests that Roflumilast will be useful in the treatment of chronic inflammatory disorders such as asthma and chronic obstructive pulmonary disease.
Influence of roflumilast on airway reactivity and apoptosis in ovalbumin-sensitized Guinea pigs.[Pubmed:25310957]
Adv Exp Med Biol. 2015;838:11-8.
Chronic inflammatory diseases, associated with airway obstruction and cough, are usually treated with bronchodilating and anti-inflammatory drugs. Inhibition of phosphodiesterases (PDE) leads to both of these effects and influences apoptosis of immune cells. In chronic obstructive pulmonary disease, Roflumilast, a selective PDE4 inhibitor, has been recently approved for pharmacotherapy. The aim of this study was to evaluate the effect of long-term administration of Roflumilast in experimental allergic inflammation in guinea pigs. Male adult guinea pigs were used in the study. There were four experimental groups sensitized with ovalbumin for 14 days and thereafter treated per os, by inhalation, and intraperitoneally for 7 days with Roflumilast or vehicle. A control group was left without sensitization. Roflumilast reduced specific airway resistance after nebulization of histamine, as measured in a double-chamber whole-body plethysmograph. This effect was confirmed in in vitro organ bath, with significant decreases in tracheal and lung smooth muscle contractility after cumulative doses of histamine. Suppression of hematological and immunological markers of inflammation and enhanced apoptosis in animals treated with Roflumilast points to the possibility of a beneficial effect of Roflumilast in allergic inflammation.
Roflumilast combined with adenosine increases mucosal hydration in human airway epithelial cultures after cigarette smoke exposure.[Pubmed:25795727]
Am J Physiol Lung Cell Mol Physiol. 2015 May 15;308(10):L1068-77.
Chronic obstructive pulmonary disease (COPD) is a growing cause of morbidity and mortality worldwide. Recent studies have shown that cigarette smoke (CS) induces cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction, which leads to airway-surface liquid (ASL) dehydration. This in turn contributes to the mucus dehydration and impaired mucociliary clearance that are seen in the chronic bronchitis form of COPD. Roflumilast is a phosphodiesterase 4 inhibitor that may improve lung function and reduce the frequency of exacerbations in patients with COPD. Although Roflumilast can affect cAMP metabolism, little is known about the downstream pharmacological effects in the airways. We hypothesized that Roflumilast would increase ASL rehydration in human bronchial epithelial cultures (HBECs) after chronic CS exposure. cAMP production was measured by Forster resonance energy transfer in HEK293T cells and by ELISA in HBECs. ASL height was measured by xz-confocal microscopy after air exposure or following HBEC exposure to freshly produced CS. Roflumilast had little effect on cAMP or ASL height when applied on its own; however, Roflumilast significantly potentiated adenosine-induced increases in cAMP and ASL height in CS-exposed HBECs. Roflumilast increased the rate of ASL height recovery in cultures after CS exposure compared with controls. In contrast, the beta2-adrenergic receptor agonists isoproterenol and salmeterol failed to increase ASL height after CS exposure. Our data suggest that Roflumilast can increase ASL hydration in CS-exposed HBECs, which is predicted to be beneficial for the treatment of mucus dehydration/mucus stasis in patients with COPD chronic bronchitis.


