Tenofovir disoproxilCAS# 201341-05-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 201341-05-1 | SDF | Download SDF |
| PubChem ID | 5481350 | Appearance | Powder |
| Formula | C19H30N5O10P | M.Wt | 519.44 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Bis(POC)-PMPA; GS 4331 | ||
| Solubility | DMSO : ≥ 38 mg/mL (73.16 mM) *"≥" means soluble, but saturation unknown. | ||
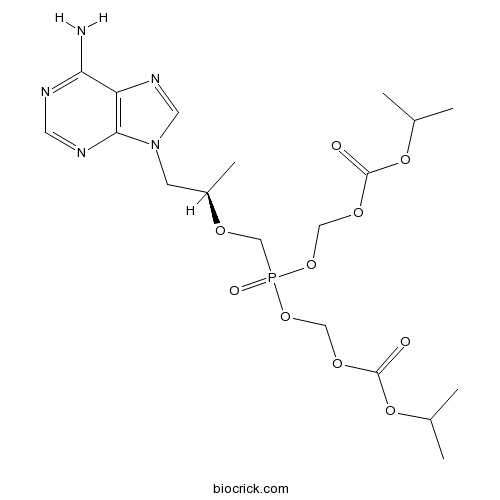
| Chemical Name | [[(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethyl-(propan-2-yloxycarbonyloxymethoxy)phosphoryl]oxymethyl propan-2-yl carbonate | ||
| SMILES | CC(C)OC(=O)OCOP(=O)(COC(C)CN1C=NC2=C1N=CN=C2N)OCOC(=O)OC(C)C | ||
| Standard InChIKey | JFVZFKDSXNQEJW-CQSZACIVSA-N | ||
| Standard InChI | InChI=1S/C19H30N5O10P/c1-12(2)33-18(25)28-9-31-35(27,32-10-29-19(26)34-13(3)4)11-30-14(5)6-24-8-23-15-16(20)21-7-22-17(15)24/h7-8,12-14H,6,9-11H2,1-5H3,(H2,20,21,22)/t14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tenofovir disoproxil fumarate monotherapy is superior to continuous add-on therapy in patients with LAM-resistant CHB with a suboptimal response to LAM plus ADV. 2. Tenofovir disoproxil has anti-HIV-1 activity. |
| Targets | HIV |

Tenofovir disoproxil Dilution Calculator

Tenofovir disoproxil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9252 mL | 9.6258 mL | 19.2515 mL | 38.503 mL | 48.1288 mL |
| 5 mM | 0.385 mL | 1.9252 mL | 3.8503 mL | 7.7006 mL | 9.6258 mL |
| 10 mM | 0.1925 mL | 0.9626 mL | 1.9252 mL | 3.8503 mL | 4.8129 mL |
| 50 mM | 0.0385 mL | 0.1925 mL | 0.385 mL | 0.7701 mL | 0.9626 mL |
| 100 mM | 0.0193 mL | 0.0963 mL | 0.1925 mL | 0.385 mL | 0.4813 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tenofovir dsoproxil is a nucleotide reverse transcriptase inhibitor to treat HIV and chronic Hepatitis B.
In Vitro:Tenofovir shows cytotoxic effects on cell viability in HK-2 cells, with IC50 values of 9.21 and 2.77 μM at 48 and 72 h in MTT assay, respectively. Tenofovir diminishes ATP levels in HK-2 cells. Tenofovir (3.0 to 28.8 μM) increases oxidative stress and protein carbonylation in HK-2 cells. Furthermore, Tenofovir induces apoptosis in HK-2 cells, and that apoptosis is induced via mitochondrial damage[1]. Tenofovir and M48U1 formulated in 0.25% HEC each inhibits the replication of both R5-tropic HIV-1BaL and X4-tropic HIV-1IIIb in activated PBMCs, and inhibits several laboratory strains and patient-derived HIV-1 isolates. The combined formulation of M48U1 and tenofovir in 0.25% HEC exhibits synergistic antiretroviral activity against infection with R5-tropic HIV-1BaL, and is not toxic to PBMCs[2].
In Vivo:Tenofovir Disoproxil Fumarate (20, 50, 140, or 300 mg/kg) administered to BLT mice, shows dose dependent activity during vaginal HIV challenge in BLT humanized mice. Tenofovir Disoproxil Fumarate (50, 140, 300 mg/kg) significantly reduces HIV transmission in BLT mice[3]. Tenofovir Disoproxil Fumarate (0.5, 1.5, or 5.0 mg/kg/day, p.o.) induces a dose-dependent decline in serum viremia in woodchucks chronically infected with WHV. Tenofovir Disoproxil Fumarate administration is safe and effective in the woodchuck model of chronic HBV infection[4].
References:
[1]. Murphy RA, et al. Establishment of HK-2 Cells as a Relevant Model to Study Tenofovir-Induced Cytotoxicity. Int J Mol Sci. 2017 Mar 1;18(3).
[2]. Musumeci G, et al. M48U1 and Tenofovir combination synergistically inhibits HIV infection in activated PBMCs and human cervicovaginal histocultures. Sci Rep. 2017 Feb 1;7:41018.
[3]. Wahl A, et al. Predicting HIV Pre-exposure Prophylaxis Efficacy for Women using a Preclinical Pharmacokinetic-Pharmacodynamic In Vivo Model. Sci Rep. 2017 Feb 1;7:41098.
[4]. Menne S, Cote PJ, Korba BE, Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob Agents Chemother. 2005 Jul;49(7):2720-8.
- Fmoc-D-Tyr(Me)-OH
Catalog No.:BCC3269
CAS No.:201335-88-8
- 1-(3,4-Dimethoxyphenyl)propane-1,2-diol
Catalog No.:BCN1507
CAS No.:20133-19-1
- H-Thr-OBzl.oxalate
Catalog No.:BCC3103
CAS No.:201274-07-9
- Diosmetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN5328
CAS No.:20126-59-4
- 3-AQC
Catalog No.:BCC6743
CAS No.:201216-42-4
- 4-PPBP maleate
Catalog No.:BCC6723
CAS No.:201216-39-9
- 2-Amino-2'-chloro-5-nitro benzophenone
Catalog No.:BCC8521
CAS No.:2011-66-7
- cis-Methylkhellactone
Catalog No.:BCN7690
CAS No.:20107-13-5
- Ravenine
Catalog No.:BCN6666
CAS No.:20105-22-0
- SB-269970
Catalog No.:BCC1927
CAS No.:201038-74-6
- Fmoc-Lys(Me)3-OH Chloride
Catalog No.:BCC3267
CAS No.:201004-29-7
- Ac-RYYRIK-NH2
Catalog No.:BCC5736
CAS No.:200959-48-4
- Rutundic acid
Catalog No.:BCN5370
CAS No.:20137-37-5
- Talarozole
Catalog No.:BCC1980
CAS No.:201410-53-9
- PKI 14-22 amide, myristoylated
Catalog No.:BCC8087
CAS No.:201422-03-9
- Boc-Dap(Boc)-OH.DCHA
Catalog No.:BCC2664
CAS No.:201472-68-6
- Fmoc-Asn-ol
Catalog No.:BCC2586
CAS No.:201484-12-0
- Dilazep dihydrochloride
Catalog No.:BCC6660
CAS No.:20153-98-4
- Deferasirox
Catalog No.:BCC3924
CAS No.:201530-41-8
- Fmoc-Pen(Trt)-OH
Catalog No.:BCC3306
CAS No.:201531-88-6
- Fmoc-D-Pen(Trt)-OH
Catalog No.:BCC3309
CAS No.:201532-01-6
- Triptocallic acid D
Catalog No.:BCN4882
CAS No.:201534-09-0
- Triptocalline A
Catalog No.:BCN6783
CAS No.:201534-10-3
- (R)-3,4-DCPG
Catalog No.:BCC7046
CAS No.:201730-10-1
Discontinuation of tenofovir disoproxil fumarate for presumed renal adverse events in treatment-naive HIV-1 patients: meta-analysis of randomized clinical studies.[Pubmed:25433663]
HIV Clin Trials. 2014 Nov-Dec;15(6):231-45.
BACKGROUND: Safety and efficacy of Tenofovir disoproxil fumarate (TDF) as a component of antiretroviral therapy (ART) have been demonstrated in clinical trials. TDF nephrotoxicity has been reported in both HIV-infected and noninfected patients. This meta-analysis explored the frequency of discontinuation attributed to renal adverse events (AEs) in randomized, controlled clinical studies that used TDF-containing regimens for ART-naive, HIV-infected patients. METHODS: A literature search of 4 electronic databases through October 31, 2013 was utilized. RCTs included were limited to randomized, prospective, comparative design in ART treatment-naive adults with HIV-1 infections receiving ART. Studies included trials containing TDF treatment regimens, with or without a non-TDF control group. Study design, follow-up, size of study population, treatment group, patient demographics, number of patients exposed to TDF or non-TDF control, baseline characteristics, investigator-defined criteria for renal AEs, and number of discontinuations due to a presumed renal AEs were extracted. RESULTS: Twenty-one clinical studies met the selection criteria. Treatment duration ranged from 48 to 288 weeks. Renal AEs led to study drug discontinuation in 44 of 10,129 patients exposed to TDF (0.43%; 95% CI, 0.32%-0.58%) and 2 of 2,013 patients exposed to non-TDF-containing regimens (0.10%; 95% CI, 0.01%-0.36%). In 5 randomized, controlled studies that included a non-TDF comparator, the estimated risk difference between the treatment groups (TDF vs non-TDF) was 0.50% (95% CI, 0.13%-0.86%; P = .007). CONCLUSIONS: In clinical studies using TDF-containing regimens, the rate of discontinuations due to renal AEs was low, but was slightly higher than in studies using non-TDF comparators.
Tenofovir disoproxil fumarate is superior to lamivudine plus adefovir in lamivudine-resistant chronic hepatitis B patients.[Pubmed:25759545]
World J Gastroenterol. 2015 Mar 7;21(9):2746-53.
AIM: To assess the efficacy of Tenofovir disoproxil fumarate (TDF) in lamivudine (LAM)-resistant patients with a suboptimal response to LAM plus adefovir (ADV). METHODS: We retrospectively analyzed the efficacy of switching to Tenofovir disoproxil fumarate in suboptimal responders to lamivudine plus adefovir. Charts were reviewed for LAM-resistant chronic hepatitis B (CHB) patients who visited the Zhejiang Province People's Hospital and The First Affiliated Hospital, College of Medicine, Zhejiang University, from June 2009 to May 2013. Patients whose serum hepatitis B virus (HBV) DNA remained detectable despite at least 6 mo of LAM plus ADV combination therapy were included. Patients with a suboptimal response to LAM plus ADV were randomized to switch to TDF monotherapy (300 mg/d orally; TDF group) or to continuation with LAM (100 mg/d orally) plus ADV (10 mg/d orally; LAM plus ADV group) and were followed for 48 wk. Serum HBV DNA was determined at baseline and weeks 4, 12, 24, 36, and 48. HBV serological markers and biochemistry were assessed at baseline and weeks 12, 24, and 48. Resistance surveillance and side effects were monitored during therapy. RESULTS: Fifty-nine patient were randomized to switch to TDF (n = 28) or continuation with LAM plus ADV (n = 31). No significant differences were found between the groups at baseline. Prior to TDF therapy, all patients had been exposed to LAM plus ADV for a median of 11 mo (range: 6-24 mo). No difference was seen in baseline serum HBV DNA between the two groups [5.13 +/- 1.08 log10 copies/mL (TDF) vs 5.04 +/- 31.16 log10 copies/mL (LAM + ADV), P = 0.639]. There was no significant difference in the rates of achieving complete virological response (CVR) at week 4 between the TDF and LAM + ADV groups (17.86% vs 6.45%, P = 0.24). The rate of achieving CVR in the TDF and LAM plus ADV groups was 75% vs 16.13% at week 12, 82.14% vs 22.58% at week 24, 89.29% vs 25.81% at week 36, and 96.43% vs 29.03% at week 48, respectively (P < 0.001). The rate of alanine aminotransferase normalization was significantly higher in the TDF than in the LAM plus ADV group at week 12 (75% vs 17.86%, P < 0.001), but not at week 24 (78.57% vs 54.84%, P = 0.097) or 48 (89.26% vs 67.74%, P = 0.062). Patients were hepatitis B e antigen (HBeAg) positive at baseline. There was no significant difference in HBeAg negativity between the TDF and LAM plus ADV groups at week 48 (4% vs 0%, P = 0.481). There were no drug-related adverse effects at week 48 in either group. CONCLUSION: Switching to TDF monotherapy was superior to continuous add-on therapy in patients with LAM-resistant CHB with a suboptimal response to LAM plus ADV.
Emtricitabine/rilpivirine/tenofovir disoproxil fumarate for the treatment of HIV-1 infection in adults.[Pubmed:26001757]
J Infect Public Health. 2015 Sep-Oct;8(5):409-17.
This paper reviews the current literature and information on the combination drug Complera() (rilpivirine/emtricitabine/Tenofovir disoproxil fumarate) that was approved by the Food and Drug Administration (FDA) in August 2011. PubMed, Cochrane and Embase (2001-2014) were searched for primary and review articles on rilpivirine, emtricitabine, and Tenofovir disoproxil fumarate, individually or in combination. Data from drug manufacturer and product label was also used. Clinical trial reports were selected, extracted and analyzed to include relevant and recent ones. Selected English-language trials were limited to those with human subjects and included both safety and efficacy outcomes. Results from two phase 3 randomized double blind trials (ECHO and THRIVE) showed that rilpivirine is non-inferior to efavirenz in suppressing viral load below 50 copies/mL in anti-retroviral therapy (ART) naive human immunodeficiency virus (HIV) infected patients. In addition, psychiatric disturbances, rash and increase in lipid levels occurred less frequently with rilpivirine when compared to efavirenz. However, virological failure and drug resistance were higher with rilpivirine in patients with baseline viral load >100,000 copies/mL. Rilpivirine showed cross resistance to efavirenz and etravirine. Efavirenz, on the other hand, did not demonstrate cross resistance to rilpivirine and etravirine, leaving the latter drugs as options for use in case of virological failure with efavirenz. Complera() remains an acceptable alternative treatment to Atripla() in ART naive patients who have a pre-ART plasma HIV RNA <100,000 copies/mL and CD4 count >200 cells/mm(3) with non-inferior efficacy and better safety and tolerability.
Effect of food on rilpivirine/emtricitabine/tenofovir disoproxil fumarate, an antiretroviral single-tablet regimen for the treatment of HIV infection.[Pubmed:24142299]
J Clin Pharmacol. 2014 Apr;54(4):378-85.
The effect of food on rilpivirine/emtricitabine/Tenofovir disoproxil fumarate single-tablet regimen (STR) was evaluated in healthy subjects. Subjects (N = 24) received rilpivirine/emtricitabine/Tenofovir disoproxil fumarate (25/200/300 mg) under fasted or fed conditions (light [390 kcal, 12 g fat]; standard [540 kcal, 21 g fat]) followed by pharmacokinetic (PK) sampling. The 90% confidence interval (CI) of the geometric mean ratio for rilpivirine, emtricitabine, tenofovir exposure was estimated for fed versus fasted dosing and light versus standard meal, with equivalence boundaries of 80 - 125%. Safety was assessed throughout study. Twenty-three subjects completed the study; one discontinued due to protocol violation. Adverse events were mild to moderate. Emtricitabine PK was unaffected. Tenofovir AUCinf was 38% and 28% higher, respectively, with standard and light meal versus fasted. Rilpivirine AUCinf and Cmax were 16% and 26% higher with a standard, and 9% and 34% with a light meal, respectively, versus fasted. Compared to standard meal, the lower limit of rilpivirine AUClast and AUCinf when taken with the light meal were narrowly below the equivalence bounds (79.9 and 79.2, respectively), rilpivirine Cmax was narrowly above (129). Rilpivirine/emtricitabine/Tenofovir disoproxil fumarate should be administered with food, which can be a standard or light meal.


