TalarozoleCytochrome P450 inhibitor CAS# 201410-53-9 |
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 201410-53-9 | SDF | Download SDF |
| PubChem ID | 9799888 | Appearance | Powder |
| Formula | C21H23N5S | M.Wt | 377.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 36 mg/mL (95.36 mM) *"≥" means soluble, but saturation unknown. | ||
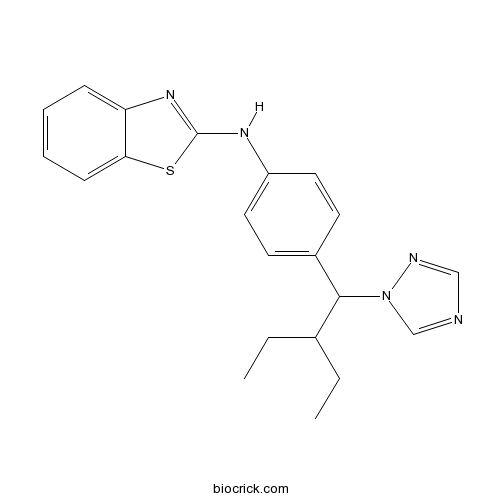
| Chemical Name | N-[4-[2-ethyl-1-(1,2,4-triazol-1-yl)butyl]phenyl]-1,3-benzothiazol-2-amine | ||
| SMILES | CCC(CC)C(C1=CC=C(C=C1)NC2=NC3=CC=CC=C3S2)N4C=NC=N4 | ||
| Standard InChIKey | SNFYYXUGUBUECJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H23N5S/c1-3-15(4-2)20(26-14-22-13-23-26)16-9-11-17(12-10-16)24-21-25-18-7-5-6-8-19(18)27-21/h5-15,20H,3-4H2,1-2H3,(H,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Talarozole is a potent and selective inhibitor of cytochrome P450. | |||||
| Targets | cytochrome P450 | |||||

Talarozole Dilution Calculator

Talarozole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6489 mL | 13.2447 mL | 26.4894 mL | 52.9787 mL | 66.2234 mL |
| 5 mM | 0.5298 mL | 2.6489 mL | 5.2979 mL | 10.5957 mL | 13.2447 mL |
| 10 mM | 0.2649 mL | 1.3245 mL | 2.6489 mL | 5.2979 mL | 6.6223 mL |
| 50 mM | 0.053 mL | 0.2649 mL | 0.5298 mL | 1.0596 mL | 1.3245 mL |
| 100 mM | 0.0265 mL | 0.1324 mL | 0.2649 mL | 0.5298 mL | 0.6622 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Talarozole is a selective inhibitor of cytochrome P450 with an IC50 at 4 nM [1].
Cytochrome P450 is concerned with oxidative metabolism of many organic chemicals of diverse structure,both in exogenous and endogenous environment. Cytochrome P450 are notable both for the diversity of reactions that they catalyze and the range of chemically dissimilar substrates upon which they act.
Talarozole is a potent and selective inhibitor of cytochrome P450 26-mediated breakdown of endogenous all-trans-retinoic acid for the treatment of psoriasis and acne. Talarozole treatment increased the mRNA expression of CRABP2, KRT4, CYP26A1 and CYP26B1 dose, meanwhile, compared with vehicle-treated skin, decreased the expression of KRT2 and IL-1α. There was no mRNA change in retinol-metabolizing enzymes. No induction of epidermal thickness or overt skin inflammation in talarozole-treated skin. Immunofluorescence analysis substantiated an upregulation of KRT4 protein, however, there were no upregulation of CYP26B1 and CYP26A1 expression was found.[1, 2]
There were 0.1% of the dose found in the skin itself after 12-24 h. The results of distribution of talarozole within the skin show that 80% was located in the epidermis, meanwhile, the remaining 20% was detected in the dermis. [3]
References:
[1] Pavez Loriè E, Cools M, Borgers M, Topical treatment with CYP26 inhibitor talarozole (R115866) dose dependently alters the expression of retinoid-regulated genes in normal human epidermis. The British Journal of Dermatology,2009,160(1):
26-36.
[2] Geria AN, Scheinfeld NS. Talarozole, a selective inhibitor of P450-mediated all-trans retinoic acid for the treatment of psoriasis and acne. Current Opinion In Investigational Drugs,2008 ,9(11):1228-1237.
[3] Baert B, De Spiegeleer B. Local skin pharmacokinetics of talarozole, a new retinoic acid metabolism-blocking agent. Skin Pharmacol Physiol,2011,24(3):151-159
- Rutundic acid
Catalog No.:BCN5370
CAS No.:20137-37-5
- Tenofovir disoproxil
Catalog No.:BCN2178
CAS No.:201341-05-1
- Fmoc-D-Tyr(Me)-OH
Catalog No.:BCC3269
CAS No.:201335-88-8
- 1-(3,4-Dimethoxyphenyl)propane-1,2-diol
Catalog No.:BCN1507
CAS No.:20133-19-1
- H-Thr-OBzl.oxalate
Catalog No.:BCC3103
CAS No.:201274-07-9
- Diosmetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN5328
CAS No.:20126-59-4
- 3-AQC
Catalog No.:BCC6743
CAS No.:201216-42-4
- 4-PPBP maleate
Catalog No.:BCC6723
CAS No.:201216-39-9
- 2-Amino-2'-chloro-5-nitro benzophenone
Catalog No.:BCC8521
CAS No.:2011-66-7
- cis-Methylkhellactone
Catalog No.:BCN7690
CAS No.:20107-13-5
- Ravenine
Catalog No.:BCN6666
CAS No.:20105-22-0
- SB-269970
Catalog No.:BCC1927
CAS No.:201038-74-6
- PKI 14-22 amide, myristoylated
Catalog No.:BCC8087
CAS No.:201422-03-9
- Boc-Dap(Boc)-OH.DCHA
Catalog No.:BCC2664
CAS No.:201472-68-6
- Fmoc-Asn-ol
Catalog No.:BCC2586
CAS No.:201484-12-0
- Dilazep dihydrochloride
Catalog No.:BCC6660
CAS No.:20153-98-4
- Deferasirox
Catalog No.:BCC3924
CAS No.:201530-41-8
- Fmoc-Pen(Trt)-OH
Catalog No.:BCC3306
CAS No.:201531-88-6
- Fmoc-D-Pen(Trt)-OH
Catalog No.:BCC3309
CAS No.:201532-01-6
- Triptocallic acid D
Catalog No.:BCN4882
CAS No.:201534-09-0
- Triptocalline A
Catalog No.:BCN6783
CAS No.:201534-10-3
- (R)-3,4-DCPG
Catalog No.:BCC7046
CAS No.:201730-10-1
- (S)-3,4-DCPG
Catalog No.:BCC7012
CAS No.:201730-11-2
- Isodiospyrin
Catalog No.:BCN4883
CAS No.:20175-84-2
Local skin pharmacokinetics of talarozole, a new retinoic acid metabolism-blocking agent.[Pubmed:21228620]
Skin Pharmacol Physiol. 2011;24(3):151-9.
Talarozole is a new highly potent and selective azole derivative which inhibits cytochrome-P450-dependent all-trans-retinoic acid catabolism. It is in clinical development for the treatment of psoriasis and acne. However, no local pharmacokinetic data on the diffusion behaviour of Talarozole in the skin itself are available. As topical application might be an interesting alternative to oral therapy because of the reduced systemic side effects, the aim of this study was to assess the transdermal behaviour of topically applied Talarozole, including its within-skin distribution. Franz diffusion cell experiments with Talarozole dissolved in different pharmaceutically relevant solvents (i.e. propylene glycol, oleolyl macrogol glyceride, isopropyl myristate, diethylene glycol monoethylether, ethanol, caprylic/capric triglyceride and caprylocaproyl macrogol glyceride) were performed to assess the transdermal behaviour of Talarozole. Talarozole slightly diffused into the skin only when dissolved in propylene glycol, isopropyl myristate or ethanol. Although only 0.1% of the dose applied was found in the skin itself after 12-24 h, this was sufficient to achieve local concentrations well above the half-maximal inhibitory concentration value for Talarozole. The distribution of Talarozole within the skin was investigated: 80% was located in the epidermis, while the remaining 20% was found in the dermis. The epidermal concentration of Talarozole achieved after a single topical application was sufficiently high to enable the potential induction of local retinoid-like effects.
Talarozole, a selective inhibitor of P450-mediated all-trans retinoic acid for the treatment of psoriasis and acne.[Pubmed:18951302]
Curr Opin Investig Drugs. 2008 Nov;9(11):1228-37.
Talarozole, being developed by Barrier Therapeutics Inc under license from Johnson & Johnson, is a potent and selective inhibitor of cytochrome P450 26-mediated breakdown of endogenous all-trans retinoic acid for the treatment of psoriasis and acne. Phase II clinical trials of an oral formulation of Talarozole in patients with psoriasis and with acne, and a phase I clinical trial of a topical formulation have been completed. At the time of publication, Barrier Therapeutics had suspended the development of Talarozole as part of a series of cost-cutting initiatives; the company had also been acquired by Stiefel Laboratories Inc. No formal announcement had been made regarding the further development of Talarozole.
Topical treatment with CYP26 inhibitor talarozole (R115866) dose dependently alters the expression of retinoid-regulated genes in normal human epidermis.[Pubmed:19016711]
Br J Dermatol. 2009 Jan;160(1):26-36.
BACKGROUND: An alternative approach to retinoid therapy is to inhibit the cytochrome P450 (CYP)-mediated catabolism of endogenous all-trans retinoic acid in the skin by applying retinoic acid metabolism blocking agents such as Talarozole (R115866). OBJECTIVES: To study the effects of topical Talarozole on retinoid biomarkers in normal skin in a randomized phase I trial. METHODS: Gels containing Talarozole (0.35% or 0.07%) and vehicle were applied once daily for 9 days on either buttock of 16 healthy volunteers. Epidermal shave biopsies (for mRNA analysis) and punch biopsies (for histology and immunofluorescence analysis) were collected from the treatment areas. Genes encoding the following were studied by quantitative real-time polymerase chain reaction: cellular retinoic acid binding protein 2 (CRABP2), cytokeratins (KRT2 and KRT4), CYP26A1, CYP26B1, CYP26C1 and CYP2S1, two enzymes in the retinol metabolism (retinal dehydrogenase-2 and retinol acyltransferase) and two proinflammatory cytokines [interleukin (IL)-1alpha and tumour necrosis factor-alpha]. RESULTS: Talarozole treatment increased the mRNA expression of CRABP2, KRT4, CYP26A1 and CYP26B1 dose dependently, and decreased the expression of KRT2 and IL-1alpha compared with vehicle-treated skin. No mRNA change in retinol-metabolizing enzymes was obtained. There was no induction of epidermal thickness or overt skin inflammation in Talarozole-treated skin. Immunofluorescence analysis confirmed an upregulation of KRT4 protein, but no upregulation of CYP26A1 and CYP26B1 expression was detected. CONCLUSIONS: Talarozole influences the biomarker pattern consistently with increased retinoic acid stimulation. The low irritancy of Talarozole at the two examined dosages is a possible advantage over topical retinoids.


