PKI 14-22 amide, myristoylatedPKA inhibitor CAS# 201422-03-9 |
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- NVP DPP 728 dihydrochloride
Catalog No.:BCC2365
CAS No.:247016-69-9
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
- DPPI 1c hydrochloride
Catalog No.:BCC2363
CAS No.:866396-34-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 201422-03-9 | SDF | Download SDF |
| PubChem ID | 71312213 | Appearance | Powder |
| Formula | C53H100N20O12 | M.Wt | 1209.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Protein kinase inhibitor-(14-22)-amide, myristoylated | ||
| Solubility | Soluble to 1 mg/ml in 30% acetonitrile / water | ||
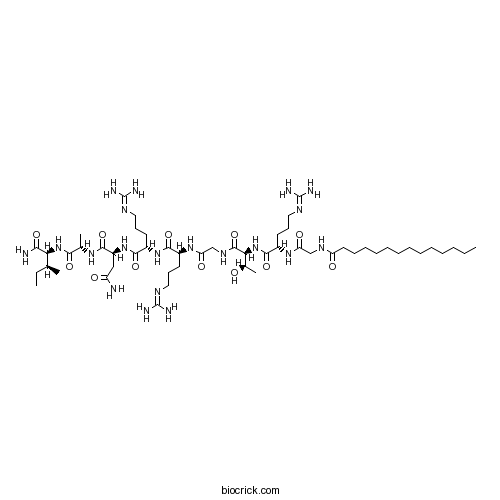
| Sequence | GRTGRRNAI (Modifications: Gly-1 = Myr-Gly, Ile-9 = C-terminal amide) | ||
| Chemical Name | (2S)-N-[(2S)-1-[[(2S,3S)-1-amino-3-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]-2-[[(2S)-5-(diaminomethylideneamino)-2-[[(2S)-5-(diaminomethylideneamino)-2-[[2-[[(2S,3R)-2-[[(2S)-5-(diaminomethylideneamino)-2-[[2-(tetradecanoylamino)acetyl]amino]pentanoyl]amino]-3-hydroxybutanoyl]amino]acetyl]amino]pentanoyl]amino]pentanoyl]amino]butanediamide | ||
| SMILES | CCCCCCCCCCCCCC(=O)NCC(=O)NC(CCCN=C(N)N)C(=O)NC(C(C)O)C(=O)NCC(=O)NC(CCCN=C(N)N)C(=O)NC(CCCN=C(N)N)C(=O)NC(CC(=O)N)C(=O)NC(C)C(=O)NC(C(C)CC)C(=O)N | ||
| Standard InChIKey | GQPQKQWUUHDDIS-JDLJUXOTSA-N | ||
| Standard InChI | InChI=1S/C53H100N20O12/c1-6-8-9-10-11-12-13-14-15-16-17-24-39(76)65-29-40(77)69-35(22-19-26-63-52(58)59)48(83)73-43(33(5)74)50(85)66-30-41(78)68-34(21-18-25-62-51(56)57)46(81)70-36(23-20-27-64-53(60)61)47(82)71-37(28-38(54)75)49(84)67-32(4)45(80)72-42(44(55)79)31(3)7-2/h31-37,42-43,74H,6-30H2,1-5H3,(H2,54,75)(H2,55,79)(H,65,76)(H,66,85)(H,67,84)(H,68,78)(H,69,77)(H,70,81)(H,71,82)(H,72,80)(H,73,83)(H4,56,57,62)(H4,58,59,63)(H4,60,61,64)/t31-,32-,33+,34-,35-,36-,37-,42-,43-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable version of protein kinase inhibitor PKI (14-22) amide. The non-myristoylated version inhibits protein kinase A (Ki = 36 nM), inhibits cell growth and induces apoptosis in human pancreatic cancer cells (PANC-1). |

PKI 14-22 amide, myristoylated Dilution Calculator

PKI 14-22 amide, myristoylated Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Myristoylated PKI (14-22) amide is an effective inhibitor of cAMP-dependent protein kinase (PKA) and blocks hyperalgesia produced by spinal administration of 8-bromo-cAMP. [1]
PKAs are the major mediators of cAMP signaling in eukaryotes. PKAs play an important role in several biological processes such as gene expression, apoptosis, tissue differentiation and cellular proliferation. PKAS play these functions through the phosphorylation of protein substrates at serine/threonine residues. [2]
PKAs play a key role in neutrophil phagocytosis. CAMP/PKAs regulate F-actin reorganization during receptor-mediated phagocytosis, particularly triggered by IgG-FcR interaction. Myristoylated PKI 14-22 amide reduced the IgG-mediated phagocytic response in a manner of dose-dependent. When the concentration is higher than 10 μM, PKI 14-22 amide can inhibit neutrophil adhesion, which make the phagocytosis measurements impossible to perform. [1]
Because the unregulated activity of PKA in mammalian cells has been implicated in the pathogenesis of several types of cancer, the development of PKI (14–22) amide has been pursued as a potential treatment for these types of cancer and many other diseases related with PKAs.[1,2]
References:
[1] Ydrenius L1, Majeed M, etal. , Activation of cAMP-dependent protein kinase is necessary for actin rearrangements in human neutrophils during phagocytosis. J Leukoc Biol. 2000 Apr;67(4):520-8.
[2] Swierczewski BE, Davies SJ. Developmental regulation of protein kinase A expression and activity in Schistosoma mansoni. Int J Parasitol. 2010 Jul;40(8):929-35.
- Talarozole
Catalog No.:BCC1980
CAS No.:201410-53-9
- Rutundic acid
Catalog No.:BCN5370
CAS No.:20137-37-5
- Tenofovir disoproxil
Catalog No.:BCN2178
CAS No.:201341-05-1
- Fmoc-D-Tyr(Me)-OH
Catalog No.:BCC3269
CAS No.:201335-88-8
- 1-(3,4-Dimethoxyphenyl)propane-1,2-diol
Catalog No.:BCN1507
CAS No.:20133-19-1
- H-Thr-OBzl.oxalate
Catalog No.:BCC3103
CAS No.:201274-07-9
- Diosmetin-7-O-beta-D-glucopyranoside
Catalog No.:BCN5328
CAS No.:20126-59-4
- 3-AQC
Catalog No.:BCC6743
CAS No.:201216-42-4
- 4-PPBP maleate
Catalog No.:BCC6723
CAS No.:201216-39-9
- 2-Amino-2'-chloro-5-nitro benzophenone
Catalog No.:BCC8521
CAS No.:2011-66-7
- cis-Methylkhellactone
Catalog No.:BCN7690
CAS No.:20107-13-5
- Ravenine
Catalog No.:BCN6666
CAS No.:20105-22-0
- Boc-Dap(Boc)-OH.DCHA
Catalog No.:BCC2664
CAS No.:201472-68-6
- Fmoc-Asn-ol
Catalog No.:BCC2586
CAS No.:201484-12-0
- Dilazep dihydrochloride
Catalog No.:BCC6660
CAS No.:20153-98-4
- Deferasirox
Catalog No.:BCC3924
CAS No.:201530-41-8
- Fmoc-Pen(Trt)-OH
Catalog No.:BCC3306
CAS No.:201531-88-6
- Fmoc-D-Pen(Trt)-OH
Catalog No.:BCC3309
CAS No.:201532-01-6
- Triptocallic acid D
Catalog No.:BCN4882
CAS No.:201534-09-0
- Triptocalline A
Catalog No.:BCN6783
CAS No.:201534-10-3
- (R)-3,4-DCPG
Catalog No.:BCC7046
CAS No.:201730-10-1
- (S)-3,4-DCPG
Catalog No.:BCC7012
CAS No.:201730-11-2
- Isodiospyrin
Catalog No.:BCN4883
CAS No.:20175-84-2
- Ac-Phe-OH
Catalog No.:BCC3005
CAS No.:2018-61-3
Activation of apolipoprotein AI gene expression by protein kinase A and kinase C through transcription factor, Sp1.[Pubmed:10829013]
J Biol Chem. 2000 Oct 13;275(41):31747-54.
Our previous finding that insulin induces apolipoprotein AI (apoAI) transcription points to the participation of intracellular signaling. This finding prompted us to ask whether two classical G-protein-coupled signaling pathways requiring activated protein kinase A (PKA) or kinase C (PKC) may also regulate apoAI. Therefore, human hepatoma, Hep G2 cells stably transfected with pAI.474-CAT, a reporter construct spanning -474 to -7 of apoAI DNA fused to chloramphenicol acetyltransferase (CAT) were treated with 10 microm forskolin (FSK) or 50 nm phorbol dibutyrate (PDBu) to activate PKA and PKC, respectively. Results showed that the apoAI promoter activity increased 4-5-fold following 24 h of treatment with either FSK or PDBu. Induction by either agent was blocked with actinomycin D but not the protein synthesis inhibitor, cycloheximide. The PKA inhibitor, PKI 14-22 amide, abrogated induction by FSK, 100 microm 8-bromo-cAMP, or 100 ng/ml cholera toxin, but it had no effect on activation via PKC. Similarly, PDBu induction was attenuated by 2 microm of the PKC inhibitor, GF109203X, but it did not affect FSK activity. Next we used deletional constructs to show that the actions of FSK and PDBu required the insulin-responsive core element (IRCE). This motif matched the consensus binding site for the transcription factor, Sp1. The binding of Sp1 to the IRCE was confirmed by gel-retardation and supershift analysis. Site-directed mutagenesis of the IRCE eliminated Sp1 action and induction by FSK or PDBu. Whereas overexpression of Sp1 enhanced basal and FSK or PDBu induced promoter activity, transfection of an antisense oligomer against Sp1 mRNA attenuated both parameters. In summary, activation of PKA or PKC increases apoAI promoter activity. The activity of both signaling pathways is mediated by the IRCE, a motif that binds the transcription factor, Sp1.
Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein.[Pubmed:2722799]
J Biol Chem. 1989 May 25;264(15):8802-10.
PKI-(5-24)-amide is a 20-residue peptide with the sequence, Thr5-Thr-Tyr-Ala-Asp-Phe-Ile-Ala-Ser-Gly-Arg-Thr-Gly-Arg-Arg-Asn-A la-Ile-His- Asp24-NH2, that corresponds to the active portion of the heat-stable inhibitor protein of cAMP-dependent protein kinase (Cheng, H.-C., Kemp, B. E., Pearson, R. B., Smith, A. J., Misconi, L., Van Patten, S. M., and Walsh, D. A. (1986) J. Biol. Chem. 261, 989-992). Amino acid residues in PKI-(5-24)-amide responsible for the potent inhibition (Ki = 2.3 nM) of the catalytic subunit of protein kinase were further investigated using deletion and substitution analogs of the synthetic peptide. Residues 5, 23, and 24 were not required for activity since the 17-residue PKI-(6-22)-amide retained full potency. Sequential removal of the first seven amino acids from the NH2 terminus of PKI-(5-24)-amide caused a progressive 50-fold loss of inhibitory potency. In contrast, substitution of either Thr6, Asp9, or Ile11 with alanine, or Ala8 by leucine, in PKI-(5-22)-amide produced less than 3-fold decreases in potency. Of the 2 aromatic residues in PKI-(5-22)-amide, the individual substitution of Phe10 and Tyr7 by alanine caused, respectively, 90- and 5-fold decreases in inhibitory potency, demonstrating important roles for each. This NH2-terminal portion of the peptide is believed to contain a significant portion of alpha-helix. Many recognition or structural determinants are also essential in the COOH-terminal portion of PKI-(5-22)-amide. In addition to the basic subsite provided by the three arginines, several other of the residues are critical for full inhibitory potency. Substitution of Ile22 by glycine in either PKI-(5-22)-amide or PKI-(14-22)-amide lowered the inhibitory potency by 150- and 50-fold, respectively. Separate replacement of Gly17 or Asn20, in either PKI-(5-22)-amide or PKI-(14-22)-amide, caused 7-15-fold decreases in potency. Substitution of both Gly17 and Asn20 together (in PKI-(14-22)-amide) produced a synergistic loss of inhibitory activity. [Leu13,Ile14]PKI-(5-22)-amide, a doubly substituted analog exhibited a 42-fold increase in Ki value. We conclude that Ser13 and/or Gly14, Gly17, Asn20, and Ile22 each contribute important features to the binding of these inhibitory peptides to the protein kinase, either by providing recognition determinants, inducing structure, and/or allowing essential peptide backbone flexibility.(ABSTRACT TRUNCATED AT 400 WORDS)
Inhibition of pancreatic cancer cell growth and induction of apoptosis with novel therapies directed against protein kinase A.[Pubmed:12947318]
Surgery. 2003 Aug;134(2):197-205.
BACKGROUND: Pancreatic cancer is the most lethal abdominal malignancy. Expression of the RIalpha subunit of protein kinase A (PKA) has been associated with neoplastic transformation and mitogenic signaling. The effect of PKA inhibition on pancreatic cancer cell growth and apoptosis is unknown. In pancreatic cancer cells, we sought to determine (1) whether inhibition of PKA can inhibit growth or induce apoptosis, and (2) whether growth can be inhibited by silencing of RIalpha expression. METHODS: Human pancreatic cancer cells (PANC-1, MIA PaCa-2, and SUIT-2) were treated with inhibitors of PKA (H89 or PKI) and cell growth, kinase activity, and induction of apoptosis measured. Small inhibitory RNA (siRNA) directed against the RIalpha subunit was synthesized and transfected into PANC-1 cells. RESULTS: H89 decreased PKA activity and inhibited pancreatic cancer cell growth. Apoptosis was also induced by H89 in PANC-1 and MIA PaCa-2 cells. PANC-1 cells express high levels of the RIalpha subunit; transfection of siRNA decreased RIalpha protein expression and inhibited growth. CONCLUSIONS: Inhibition of PKA in pancreatic cancer cells induces growth arrest and apoptosis; similar effects are noted in cells with siRNA used to block RIalpha expression. Inhibition of PKA may represent a novel therapeutic strategy for the adjuvant treatment of pancreatic cancer.


