TerpineolCAS# 98-55-5 |
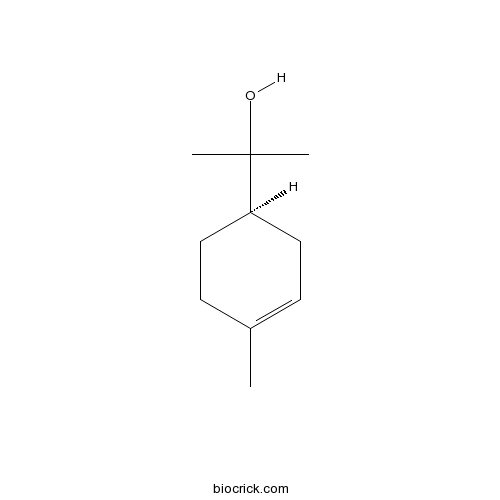
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 98-55-5 | SDF | Download SDF |
| PubChem ID | 442501 | Appearance | Colorless liquid |
| Formula | C10H18O | M.Wt | 154.3 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Synonyms | p-Menth 1-ene 8-ol | ||
| Solubility | DMSO : ≥ 250 mg/mL (1620.75 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[(1R)-4-methylcyclohex-3-en-1-yl]propan-2-ol | ||
| SMILES | CC1=CCC(CC1)C(C)(C)O | ||
| Standard InChIKey | WUOACPNHFRMFPN-VIFPVBQESA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-8-4-6-9(7-5-8)10(2,3)11/h4,9,11H,5-7H2,1-3H3/t9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | α-Terpineol possesses antifungal activity against Trichophyton mentagrophytes, it also exhibits strong antimicrobial activity against periodontopathic and cariogenic bacteria. α-Terpineol shows anticonvulsant, and anti-inflammatory activities, it inhibits the gene expression of the IL-6 receptor. |
| Targets | IL Receptor | Antifection |
| In vitro | Effect of citral, eugenol, nerolidol and alpha-terpineol on the ultrastructural changes of Trichophyton mentagrophytes.[Pubmed: 19345255 ]Fitoterapia. 2009 Jul;80(5):290-6.The antifungal effects of citral, eugenol, nerolidol and alpha-Terpineol on Trichophyton mentagrophytes were investigated. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria.[Pubmed: 22537719 ]Anaerobe. 2012 Jun;18(3):369-72.
|
| In vivo | Evolution of the Anticonvulsant Activity of α-Terpineol[Reference: WebLink]Pharmaceutical Biology, 2007 , 45 (1) :69-70.α-Terpineol, a monoterpenoid alcohol, was investigated for its anticonvulsant activity. |
| Kinase Assay | Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells.[Pubmed: 17867636 ]J Agric Food Chem. 2007 Oct 3;55(20):8040-6.
|
| Cell Research | Synergistic Effect and Mechanism of Cineole and Terpineol on In-vitro Transdermal Delivery of Huperzine A from Microemulsions.[Pubmed: 24250600]Iran J Pharm Res. 2013 Spring;12(2):271-80.The aim of the present study was to investigate the influence and the mechanisms of cineole and Terpineol on the in-vitro transdermal delivery of huperzine A from microemulsions, and their potential synergistic effect on the permeation enhancement. |

Terpineol Dilution Calculator

Terpineol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4809 mL | 32.4044 mL | 64.8088 mL | 129.6176 mL | 162.022 mL |
| 5 mM | 1.2962 mL | 6.4809 mL | 12.9618 mL | 25.9235 mL | 32.4044 mL |
| 10 mM | 0.6481 mL | 3.2404 mL | 6.4809 mL | 12.9618 mL | 16.2022 mL |
| 50 mM | 0.1296 mL | 0.6481 mL | 1.2962 mL | 2.5924 mL | 3.2404 mL |
| 100 mM | 0.0648 mL | 0.324 mL | 0.6481 mL | 1.2962 mL | 1.6202 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Aminophenylarsonic acid
Catalog No.:BCC8688
CAS No.:98-50-0
- Benzenesulfonic acid
Catalog No.:BCC8846
CAS No.:98-11-3
- Methyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN1294
CAS No.:97966-29-5
- Leachianone A
Catalog No.:BCN4530
CAS No.:97938-31-3
- Sophoraflavanone G
Catalog No.:BCN2987
CAS No.:97938-30-2
- Methyl 3-carbazolecarboxylate
Catalog No.:BCN4529
CAS No.:97931-41-4
- 3,4'-Dihydroxy-3,5',7-trimethoxyflavan
Catalog No.:BCN4528
CAS No.:97914-19-7
- Estradiol valerate
Catalog No.:BCC4482
CAS No.:979-32-8
- Norfloxacin lactate
Catalog No.:BCC9104
CAS No.:97867-34-0
- 8,9-Didehydro-7-hydroxydolichodial
Catalog No.:BCN6674
CAS No.:97856-19-4
- Penciclovir Sodium
Catalog No.:BCC5635
CAS No.:97845-62-0
- IMD 0354
Catalog No.:BCC4556
CAS No.:978-62-1
- H-Pyr-OH
Catalog No.:BCC3328
CAS No.:98-79-3
- Acetophenone
Catalog No.:BCN8300
CAS No.:98-86-2
- Nicotinamide
Catalog No.:BCN1025
CAS No.:98-92-0
- Pyrazinamide
Catalog No.:BCC4932
CAS No.:98-96-4
- Brompheniramine hydrogen maleate
Catalog No.:BCC4515
CAS No.:980-71-2
- Lomefloxacin HCl
Catalog No.:BCC4673
CAS No.:98079-52-8
- Lupinalbin A
Catalog No.:BCN8191
CAS No.:98094-87-2
- Ailanthone
Catalog No.:BCN4531
CAS No.:981-15-7
- Difloxacin HCl
Catalog No.:BCC3764
CAS No.:98106-17-3
- 5,2',6'-Trihydroxy-6,7,8-trimethoxyflavone
Catalog No.:BCN1293
CAS No.:98187-98-5
- Eltoprazine hydrochloride
Catalog No.:BCC5422
CAS No.:98224-03-4
- Eltoprazine
Catalog No.:BCC5421
CAS No.:98206-09-8
Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells.[Pubmed:17867636]
J Agric Food Chem. 2007 Oct 3;55(20):8040-6.
Epithelial buccal cells (KB) were exposed to orange juice or orange juice fractions containing either the dry matter (DM), the volatile compounds (aqueous distillate, AD), or individual nonvolatile or volatile components. Intracellular formation of the pro-inflammatory cytokine IL-6 was analyzed by flow cytometry. Exposure to whole orange juice resulted in an increase in IL-6 formation of 23% compared to nontreated control cells, whereas treatment of the cells with either DM or AD resulted in a 22 or 1% increase, respectively. Dose-response experiments revealed that exposure of the cells to a 2- or 4-fold concentrated AD resulted in an increased IL-6 formation, whereas an inhibiting effect was measured after treatment of the cells with an 8-fold concentrated AD. These results indicated the presence of pro- as well as anti-inflammatory compounds in the aqueous distillate. To identify the active principles, volatile compounds present in the AD-treated cells were analyzed by GC-MS. In particular, limonene, linalool, and alpha-Terpineol were shown to be present in significant amounts. Subsequent studies on the IL-6 formation revealed that limonene had a stimulating effect and alpha-Terpineol had an inhibiting effect, whereas linalool had no effect. This anti-inflammatory effect of alpha-Terpineol on IL-6 formation was verified by quantitative real-time reverse transcription Polymerase Chain Reaction experiments in which alpha-Terpineol inhibited the gene expression of the IL-6 receptor.
Synergistic Effect and Mechanism of Cineole and Terpineol on In-vitro Transdermal Delivery of Huperzine A from Microemulsions.[Pubmed:24250600]
Iran J Pharm Res. 2013 Spring;12(2):271-80.
The aim of the present study was to investigate the influence and the mechanisms of cineole and Terpineol on the in-vitro transdermal delivery of huperzine A from microemulsions, and their potential synergistic effect on the permeation enhancement. The transdermal delivery of huperzine A from microemulsions with different concentrations of cineole and Terpineol through the rat abdominal skin was determined by Franz-type diffusion cells. The partition coefficient of huperzine A between the full thickness skin and microemulsion was determined. Attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) was carried out to analyze the effects of cineole and Terpineol on the biophysical properties of the stratum corneum (SC) and the mechanisms of permeation enhancement. These results indicated that cineole and Terpineol could synergistically increase the transdermal delivery of huperzine A from microemulsions through increasing the partition and diffusion coefficients of huperzine A. ATR-FTIR studies further validated the synergistic effect and revealed that the enhancing mechanisms were due to increasing the disorderliness and fluidity of SC lipid alkyl chains, disrupting the structure of keratin in SC, and extracting SC lipids. In conclusion, cineole and Terpineol, acting synergistically to enhance the transdermal delivery of huperzine A from microemulsions, might provide an alternative permeation enhancer combination for the transdermal delivery of huperzine A.
Antimicrobial effect of linalool and alpha-terpineol against periodontopathic and cariogenic bacteria.[Pubmed:22537719]
Anaerobe. 2012 Jun;18(3):369-72.
Linalool and alpha-Terpineol exhibited strong antimicrobial activity against periodontopathic and cariogenic bacteria. However, their concentration should be kept below 0.4 mg/ml if they are to be used as components of toothpaste or gargling solution. Moreover, other compounds with antimicrobial activity against periodontopathic and cariogenic bacteria should be used in combination.
Effect of citral, eugenol, nerolidol and alpha-terpineol on the ultrastructural changes of Trichophyton mentagrophytes.[Pubmed:19345255]
Fitoterapia. 2009 Jul;80(5):290-6.
The antifungal effects of citral, eugenol, nerolidol and alpha-Terpineol on Trichophyton mentagrophytes were investigated. Citral over 0.1 mg/ml strongly inhibited the hyphal growth of T. mentagrophytes, and the antifungal activity of alpha-Terpineol was less effective. The morphological changes of the fungus exposed to the terpenes were observed by electron microscopy. The hyphae were distorted and collapsed at 0.2, 0.4 and 1 mg/ml of eugenol, nerolidol and alpha-Terpineol respectively, and cell membrane and organelles were irreversibly damaged at 0.2 mg/ml citral. These suggested that four terpenes possess antifungal activity against T. mentagrophytes, and the activity might lead to irreversible cellular disruption.


