Tolfenamic AcidNon-steroidal anti-inflammatory drug CAS# 13710-19-5 |
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- Entacapone
Catalog No.:BCC2217
CAS No.:130929-57-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13710-19-5 | SDF | Download SDF |
| PubChem ID | 5507 | Appearance | Powder |
| Formula | C14H12ClNO2 | M.Wt | 261.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (382.12 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
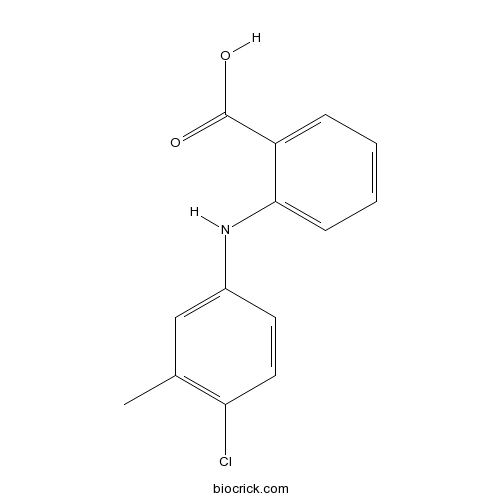
| Chemical Name | 2-(4-chloro-3-methylanilino)benzoic acid | ||
| SMILES | CC1=C(C=CC(=C1)NC2=CC=CC=C2C(=O)O)Cl | ||
| Standard InChIKey | QDNMBJXNLJFNHT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12ClNO2/c1-9-8-10(6-7-12(9)15)16-13-5-3-2-4-11(13)14(17)18/h2-8,16H,1H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tolfenamic acid (TA) is one of the class of non-steroidal anti-inflammatory drugs (NSAIDs).
Target: COX-2
Tolfenamic acid is a NSAID. Tolfenamic acid treatment inhibited cell growth and induced apoptosis as measured by caspase activity and bioelectric impedance. Tolfenamic acid induced EGR-1 expression at the transcription level, and analysis of the EGR-1 promoter showed that a putative ETS-binding site, located at ?400 and ?394 bp, was required for activation by tolfenamic acid [1].
Tolfenamic acid preferentially inhibited COX-2, with meloxicam inhibiting COX-2 activity 12 times more effectively than COX-1 activity. Carprofen was only 1.75 times more selective for COX-2 than for COX-1, and ketoprofen was slightly more selective for COX-1 [2]. References: | |||||

Tolfenamic Acid Dilution Calculator

Tolfenamic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8212 mL | 19.1058 mL | 38.2117 mL | 76.4234 mL | 95.5292 mL |
| 5 mM | 0.7642 mL | 3.8212 mL | 7.6423 mL | 15.2847 mL | 19.1058 mL |
| 10 mM | 0.3821 mL | 1.9106 mL | 3.8212 mL | 7.6423 mL | 9.5529 mL |
| 50 mM | 0.0764 mL | 0.3821 mL | 0.7642 mL | 1.5285 mL | 1.9106 mL |
| 100 mM | 0.0382 mL | 0.1911 mL | 0.3821 mL | 0.7642 mL | 0.9553 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tolfenamic acid (TA) is one of the class of non-steroidal anti-inflammatory drugs (NSAIDs).Tolfenamic acid is a NSAID. Tolfenamic acid treatment inhibited cell growth and induced apoptosis as measured by caspase activity and bioelectric impedance. Tolfena
- Pimecrolimus
Catalog No.:BCC4703
CAS No.:137071-32-0
- Alprenolol hydrochloride
Catalog No.:BCC7490
CAS No.:13707-88-5
- PACAP 1-38
Catalog No.:BCC6962
CAS No.:137061-48-4
- Walsuralactam A
Catalog No.:BCN6734
CAS No.:1370556-82-3
- Episyringaresinol 4'-O-β-D-glncopyranoside
Catalog No.:BCC8957
CAS No.:137038-13-2
- Pseudoginsenoside Rh2
Catalog No.:BCC8353
CAS No.:1370264-16-6
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- 3',4'-Di-O-acetyl-2',6'-di-O-p-coumaroylastragalin
Catalog No.:BCN6610
CAS No.:137018-33-8
- Amprolium HCl
Catalog No.:BCC4626
CAS No.:137-88-2
- L-Ascorbyl 6-palmitate
Catalog No.:BCC4915
CAS No.:137-66-6
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Calcium pantothenate
Catalog No.:BCN8503
CAS No.:137-08-6
- (±)-Marmesin
Catalog No.:BCN3618
CAS No.:13710-70-8
- BETP
Catalog No.:BCC6286
CAS No.:1371569-69-5
- Toddalosin
Catalog No.:BCN6194
CAS No.:137182-37-7
- Spinorphin
Catalog No.:BCC2349
CAS No.:137201-62-8
- Sodium Orthovanadate
Catalog No.:BCC3856
CAS No.:13721-39-6
- Voriconazole
Catalog No.:BCC2275
CAS No.:137234-62-9
- Dodoviscin A
Catalog No.:BCN3927
CAS No.:1372527-25-7
- Dodoviscin H
Catalog No.:BCN3918
CAS No.:1372527-39-3
- Dodoviscin I
Catalog No.:BCN3926
CAS No.:1372527-40-6
- Dodoviscin J
Catalog No.:BCN3945
CAS No.:1372527-42-8
- GSK2636771
Catalog No.:BCC4993
CAS No.:1372540-25-4
- Chlorantholide A
Catalog No.:BCN4835
CAS No.:1372558-33-2
Rationally Developed Organic Salts of Tolfenamic Acid and Its beta-Alanine Derivatives for Dual Purposes as an Anti-Inflammatory Topical Gel and Anticancer Agent.[Pubmed:28150904]
Chem Asian J. 2017 Apr 4;12(7):792-803.
A new series of primary ammonium monocarboxylate (PAM) salts of a nonsteroidal anti-inflammatory drug (NSAID), namely, Tolfenamic Acid (TA), and its beta-alanine derivatives were generated. Nearly 67 % of the salts in the series showed gelling abilities with various solvents, including water (biogenic solvent) and methyl salicylate (typically used for topical gel formulations). Gels were characterized by rheology, electron microscopy, and so forth. Structure-property correlations based on single-crystal and powder XRD data of several gelator and nongelator salts revealed intriguing insights. Studies (in vitro) on an aggressive human breast cancer cell line (MDA-MB-231) with the l-tyrosine methyl ester salt of TA (S7) revealed that the hydrogelator salt was more effective at killing cancer cells than the mother drug TA (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay); displayed better anti-inflammatory activity compared with that of TA (prostaglandin E2 assay); could be internalized within the cancer cells, as revealed by fluorescence microscopy; and inhibited effectively migration of the cancer cells. Thus, the easily accessible ambidextrous gelator salt S7 can be used for two purposes: as an anti-inflammatory topical gel and as an anticancer agent.
Targeting specificity protein 1 transcription factor and survivin using tolfenamic acid for inhibiting Ewing sarcoma cell growth.[Pubmed:28025760]
Invest New Drugs. 2017 Apr;35(2):158-165.
Transcription factor Specificity protein 1 (Sp1) and its downstream target survivin (inhibitor of apoptosis protein), play major roles in the pathogenesis of various cancers. Ewing Sarcoma (ES) is a common soft tissue/bone tumor in adolescent and young adults. Overexpression of survivin is also linked to the aggressiveness and poor prognosis of ES. Small molecule Tolfenamic Acid (TA) inhibits Sp1 and survivin in cancer cells. In this investigation, we demonstrate a strategy to target Sp1 and survivin using TA and positive control Mithramycin A (Mit) to inhibit ES cell growth. Knock down of Sp1 using small interfering RNA (siRNA) resulted in significant (p < 0.05) inhibition of CHLA-9 and TC-32 cell growth as assessed by CellTiter-Glo assay kit. TA or Mit treatment caused dose/time-dependent inhibition of cell viability, and this inhibition was correlated with a decrease in Sp1 and survivin protein levels in ES cells. Quantitative PCR results showed that Mit treatment decreased the mRNA expression of both survivin and Sp1, whereas TA diminished only survivin but not Sp1. Proteasome inhibitor restored TA-induced inhibition of Sp1 protein expression suggesting that TA might cause proteasome-dependent degradation. Gel shift assay using ES cell nuclear extract and biotinylated Sp1 consensus oligonucleotides confirmed that both TA and Mit decreased DNA-binding activity of Sp1. These results demonstrate that both Mit and TA reduce expression of Sp1 and survivin, disrupt Sp1 DNA-binding and inhibit ES cell proliferation. This investigation suggests that targeting Sp1 and survivin could be an effective strategy for inhibiting ES cell growth.
Drug Repurposing: Tolfenamic Acid Inactivates PrbP, a Transcriptional Accessory Protein in Liberibacter asiaticus.[Pubmed:27803694]
Front Microbiol. 2016 Oct 18;7:1630.
CLIBASIA_01510, PrbP, is a predicted RNA polymerase binding protein in Liberibacter asiaticus. PrbP was found to regulate expression of a small subset of ribosomal genes through interactions with the beta-subunit of the RNA polymerase and a short, specific sequence on the promoter region. Molecular screening assays were performed to identify small molecules that interact with PrbP in vitro. Chemical hits were analyzed for therapeutic efficacy against L. asiaticus via an infected leaf assay, where the transcriptional activity of L. asiaticus was found to decrease significantly after exposure to Tolfenamic Acid. Similarly, Tolfenamic Acid was found to inhibit L. asiaticus infection in highly symptomatic citrus seedlings. Our results indicate that PrbP is an important transcriptional regulator for survival of L. asiaticus in planta, and the chemicals identified by molecular screening assays could be used as a therapeutic treatment for huanglongbing disease.
Tolfenamic acid-induced alterations in genes and pathways in pancreatic cancer cells.[Pubmed:28099934]
Oncotarget. 2017 Feb 28;8(9):14593-14603.
Non-steroidal anti-inflammatory drugs (NSAIDs) are being tested extensively for their role in the treatment and prevention of several cancers. Typically NSAIDs exhibit anti-tumor activities via modulation of cyclooxygenase (COX)-dependent mechanisms, however, an anti-cancer NSAID Tolfenamic Acid (TA) is believed to work through COX-independent pathways. Results from our laboratory and others have demonstrated the anti-cancer activity of TA in various cancer models including pancreatic cancer. TA has been shown to modulate certain cellular processes including, apoptosis, reactive oxygen species and signaling. In this study, molecular profiling was performed to precisely understand the mode of action of TA. Three pancreatic cancer cell lines, L3.6pl, MIA PaCa-2, and Panc1 were treated with TA (50 muM for 48 h) and the changes in gene expression was evaluated using the Affymetrix GeneChip Human Gene ST Array platform. Microarray results were further validated using quantitative PCR for seven genes altered by TA treatment in all three cell lines. Functional analysis of differentially expressed genes (2 fold increase or decrease, p < 0.05) using Ingenuity Pathway Analysis software, revealed that TA treatment predominantly affected the genes involved in cell cycle, cell growth and proliferation, and cell death and survival. Promoter analysis of the differentially expressed genes revealed that they are enriched for Sp1 binding sites, suggesting that Sp1 could be a major contributor in mediating the effect of TA. The gene expression studies identified new targets involved in TA's mode of action, while supporting the hypothesis about the association of Sp1 in TA mediated effects in pancreatic cancer.


