VCH-916HCV NS5B polymerase inhibitor CAS# 1200133-34-1 |
- Anguizole
Catalog No.:BCC1365
CAS No.:442666-98-0
- Asunaprevir (BMS-650032)
Catalog No.:BCC1374
CAS No.:630420-16-5
- Balapiravir
Catalog No.:BCC1396
CAS No.:690270-29-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1200133-34-1 | SDF | Download SDF |
| PubChem ID | 46930992 | Appearance | Powder |
| Formula | C26H36KNO4S | M.Wt | 497.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 39 mg/mL (78.36 mM) *"≥" means soluble, but saturation unknown. | ||
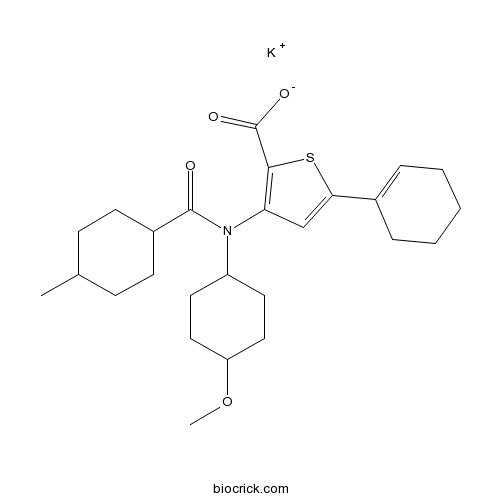
| Chemical Name | potassium;5-(cyclohexen-1-yl)-3-[(4-methoxycyclohexyl)-(4-methylcyclohexanecarbonyl)amino]thiophene-2-carboxylate | ||
| SMILES | CC1CCC(CC1)C(=O)N(C2CCC(CC2)OC)C3=C(SC(=C3)C4=CCCCC4)C(=O)[O-].[K+] | ||
| Standard InChIKey | RYXIBQLRUHDYEE-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C26H37NO4S.K/c1-17-8-10-19(11-9-17)25(28)27(20-12-14-21(31-2)15-13-20)22-16-23(32-24(22)26(29)30)18-6-4-3-5-7-18;/h6,16-17,19-21H,3-5,7-15H2,1-2H3,(H,29,30);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | VCH-916 is a novel nonnucleoside HCV NS5B polymerase inhibitor.
IC50 Value:
Target: HCV
VCH-916 is a novel allosteric inhibitor of HCV NS5B polymerase. The RNA-dependent RNA polymerase (NS5B) of HCV is one of the attractive validated targets for development of new drugs to block HCV infection. VCH-916 is currently being evaluated for safety/tolerability, pharmacokinetics and anti-viral efficacy in chronically infected HCV patient. References: | |||||

VCH-916 Dilution Calculator

VCH-916 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0091 mL | 10.0456 mL | 20.0912 mL | 40.1824 mL | 50.228 mL |
| 5 mM | 0.4018 mL | 2.0091 mL | 4.0182 mL | 8.0365 mL | 10.0456 mL |
| 10 mM | 0.2009 mL | 1.0046 mL | 2.0091 mL | 4.0182 mL | 5.0228 mL |
| 50 mM | 0.0402 mL | 0.2009 mL | 0.4018 mL | 0.8036 mL | 1.0046 mL |
| 100 mM | 0.0201 mL | 0.1005 mL | 0.2009 mL | 0.4018 mL | 0.5023 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
VCH-916 is a novel allosteric inhibitor of HCV NS5B polymerase. The RNA-dependent RNA polymerase (NS5B) of HCV is one of the attractive validated targets for development of new drugs to block HCV infection. VCH-916 is currently being evaluated for safety/tolerability, pharmacokinetics and anti-viral efficacy in chronically infected HCV patient.
- Donepezil HCl
Catalog No.:BCC4569
CAS No.:120011-70-3
- Dichlorphenamide
Catalog No.:BCC3761
CAS No.:120-97-8
- 1,2-Benzenediol
Catalog No.:BCN6103
CAS No.:120-80-9
- N,N'-Bis(salicylidene)-1,3-propanediamine
Catalog No.:BCC9063
CAS No.:120-70-7
- 2'-Methylacetanilide
Catalog No.:BCC8581
CAS No.:120-66-1
- Isosafrole
Catalog No.:BCC3976
CAS No.:120-58-1
- Benzyl benzoate
Catalog No.:BCN8521
CAS No.:120-51-4
- Ethylparaben
Catalog No.:BCN6094
CAS No.:120-47-8
- Desoxyanisoin
Catalog No.:BCN2264
CAS No.:120-44-5
- 3-Amino-4-methoxybenzanilide
Catalog No.:BCC8613
CAS No.:120-35-4
- Clorofene
Catalog No.:BCC8919
CAS No.:120-32-1
- Tropine benzilate
Catalog No.:BCN1921
CAS No.:3736-36-5
- Edgeworin
Catalog No.:BCN6561
CAS No.:120028-43-5
- Meridinol
Catalog No.:BCN6087
CAS No.:120051-54-9
- Shizukanolide F
Catalog No.:BCN6411
CAS No.:120061-96-3
- CRF (6-33)
Catalog No.:BCC5791
CAS No.:120066-38-8
- Fmoc-D-Arg(Mtr)-OH
Catalog No.:BCC3078
CAS No.:120075-24-3
- Flavanthrin
Catalog No.:BCN3687
CAS No.:120090-80-4
- IPI-145 (INK1197)
Catalog No.:BCC1104
CAS No.:1201438-56-3
- Crotaleschenine
Catalog No.:BCN2077
CAS No.:120154-95-2
- Vinflunine Tartrate
Catalog No.:BCC4602
CAS No.:1201898-17-0
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- TCS 2210
Catalog No.:BCC7798
CAS No.:1201916-31-5
- Cynoglossamine
Catalog No.:BCN1970
CAS No.:120193-39-7
Current race in the development of DAAs (direct-acting antivirals) against HCV.[Pubmed:24735613]
Biochem Pharmacol. 2014 Jun 15;89(4):441-52.
The direct-acting antivirals (DAAs) currently in development for treatment of hepatitis C fall into four categories: (i) NS3/4A protease inhibitors: ABT-450/r, faldaprevir, asunaprevir, GS-9256, vedroprevir (GS-9451), danoprevir, MK-5172, vaniprevir, sovaprevir, ACH-2684, narlaprevir and simeprevir, in addition to those that are already developed [telaprevir (Incivek(R)) and boceprevir (Victrelis(R))], (ii) NS5A protein inhibitors: ABT-267, daclatasvir, ledipasvir, ACH-2928, ACH-3102, PPI-668, AZD-7295, MK-8742, and GSK 2336805; (iii) NS5B (nucleoside-type) polymerase inhibitors: sofosbuvir (now approved by the FDA since 6 December 2013), GS-0938, mericitabine, VX-135, ALS 2158 and TMC 649128; (iv) NS5B (non-nucleoside-type) polymerase inhibitors: VX-222, ABT-072, ABT-333, deleobuvir, tegobuvir, setrobuvir, VCH-916, VCH-759, BMS-791325 and TMC-647055. Future drug combinations will likely exist of two or more DAAs belonging to any of the 4 categories, with the aim to achieve (i) pan-genotypic hepatitis C virus (HCV) activity, (ii) little or no risk for resistance; (iii) short duration (i.e. 12 weeks) of treatment, and (iv) a sustained viral response (SVR) and definite cure of the disease.


