VU0152100M4 receptor allosteric modulators CAS# 409351-28-6 |
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- Ifenprodil Tartrate
Catalog No.:BCC4589
CAS No.:23210-58-4
- VU 0364770
Catalog No.:BCC4597
CAS No.:61350-00-3
- MK-801 (Dizocilpine)
Catalog No.:BCC4591
CAS No.:77086-21-6
- Latrepirdine
Catalog No.:BCC4541
CAS No.:97657-92-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 409351-28-6 | SDF | Download SDF |
| PubChem ID | 864492 | Appearance | Powder |
| Formula | C18H19N3O2S | M.Wt | 341.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ML 108 | ||
| Solubility | DMSO : ≥ 50 mg/mL (146.44 mM) *"≥" means soluble, but saturation unknown. | ||
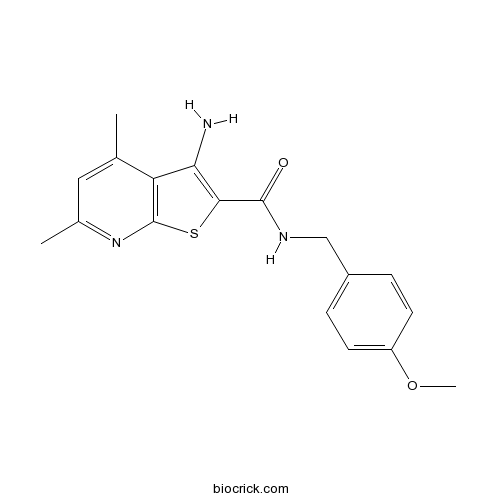
| Chemical Name | 3-amino-N-[(4-methoxyphenyl)methyl]-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide | ||
| SMILES | CC1=CC(=NC2=C1C(=C(S2)C(=O)NCC3=CC=C(C=C3)OC)N)C | ||
| Standard InChIKey | MDNWGCQSCGNTKH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19N3O2S/c1-10-8-11(2)21-18-14(10)15(19)16(24-18)17(22)20-9-12-4-6-13(23-3)7-5-12/h4-8H,9,19H2,1-3H3,(H,20,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective positive allosteric modulator of M4 muscarinic acetylcholine receptors (mAChRs) (EC50 = 380 nM). Induces 21-fold shift in ACh potency at M4 receptor. Displays no activity at other mAChR subtypes. |

VU0152100 Dilution Calculator

VU0152100 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9289 mL | 14.6443 mL | 29.2886 mL | 58.5772 mL | 73.2215 mL |
| 5 mM | 0.5858 mL | 2.9289 mL | 5.8577 mL | 11.7154 mL | 14.6443 mL |
| 10 mM | 0.2929 mL | 1.4644 mL | 2.9289 mL | 5.8577 mL | 7.3221 mL |
| 50 mM | 0.0586 mL | 0.2929 mL | 0.5858 mL | 1.1715 mL | 1.4644 mL |
| 100 mM | 0.0293 mL | 0.1464 mL | 0.2929 mL | 0.5858 mL | 0.7322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
VU0152100 is a selective and positive allosteric modulators of M4 receptor with the EC50 value of 380±93nM[1].
VU0152100 has shown a potent positive allosteric modulators that increase the response of the M4 receptor to the endogenous agonist ACh in in vitro study. In addition, VU0152100 has been reported to dese-dependently potentiate the response to the agonist (ACh) with the EC50 value of 1.9±0.2μM, and increase the maximal response to ACh to approximately 130%. Furthermore, VU0152100 has been revealed to enhance receptor activation by increasing the affinity of M4 for acetylcholine. Apart from these, VU0152100 has been found to induce a 20 to 25-fold leftward shift in the potency of ACh to displace [3H]NMS binding to M4 receptor with the Ki value from 252±19.7nM to 12.2±0.49nM [1].
References:
[1] Brady AE1, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008 Dec;327(3):941-53.
- JNJ 16259685
Catalog No.:BCC7332
CAS No.:409345-29-5
- 3,4-Dimethoxycinnamyl alcohol
Catalog No.:BCN6499
CAS No.:40918-90-9
- Sinoacutine
Catalog No.:BCN5461
CAS No.:4090-18-0
- H-Tyr-OEt.HCl
Catalog No.:BCC3125
CAS No.:4089-07-0
- 3,5-Dihydroxy-1,7-bis(3,4-dihydroxyphenyl)heptane
Catalog No.:BCN7494
CAS No.:408324-01-6
- 3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane
Catalog No.:BCN7165
CAS No.:408324-00-5
- Suprofen
Catalog No.:BCC4943
CAS No.:40828-46-4
- MDL 72222
Catalog No.:BCC6733
CAS No.:40796-97-2
- H-D-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2900
CAS No.:4079-64-5
- PD 146176
Catalog No.:BCC7504
CAS No.:4079-26-9
- FPL 55712
Catalog No.:BCC7310
CAS No.:40785-97-5
- 1,7-Diepi-8,15-cedranediol
Catalog No.:BCN5460
CAS No.:40768-81-8
- Taxiresinol
Catalog No.:BCN4637
CAS No.:40951-69-7
- Glycitein
Catalog No.:BCN5896
CAS No.:40957-83-3
- Medioresinol
Catalog No.:BCN5462
CAS No.:40957-99-1
- Desacetylcinobufotalin
Catalog No.:BCC8166
CAS No.:4099-30-3
- H-D-Ala-OBzl.TosOH
Catalog No.:BCC2850
CAS No.:41036-32-2
- Palovarotene
Catalog No.:BCC4185
CAS No.:410528-02-8
- Sirtinol
Catalog No.:BCC2224
CAS No.:410536-97-9
- Timosaponin A3
Catalog No.:BCN4999
CAS No.:41059-79-4
- Lipiferolide
Catalog No.:BCN5463
CAS No.:41059-80-7
- Neobavaisoflavone
Catalog No.:BCN3194
CAS No.:41060-15-5
- Skullcapflavone I
Catalog No.:BCN5464
CAS No.:41060-16-6
- Steviolbioside
Catalog No.:BCN6800
CAS No.:41093-60-1
Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100.[Pubmed:24442096]
Neuropsychopharmacology. 2014 Jun;39(7):1578-93.
Accumulating evidence suggests that selective M4 muscarinic acetylcholine receptor (mAChR) activators may offer a novel strategy for the treatment of psychosis. However, previous efforts to develop selective M4 activators were unsuccessful because of the lack of M4 mAChR subtype specificity and off-target muscarinic adverse effects. We recently developed VU0152100, a highly selective M4 positive allosteric modulator (PAM) that exerts central effects after systemic administration. We now report that VU0152100 dose-dependently reverses amphetamine-induced hyperlocomotion in rats and wild-type mice, but not in M4 KO mice. VU0152100 also blocks amphetamine-induced disruption of the acquisition of contextual fear conditioning and prepulse inhibition of the acoustic startle reflex. These effects were observed at doses that do not produce catalepsy or peripheral adverse effects associated with non-selective mAChR agonists. To further understand the effects of selective potentiation of M4 on region-specific brain activation, VU0152100 alone and in combination with amphetamine were evaluated using pharmacologic magnetic resonance imaging (phMRI). Key neural substrates of M4-mediated modulation of the amphetamine response included the nucleus accumbens (NAS), caudate-putamen (CP), hippocampus, and medial thalamus. Functional connectivity analysis of phMRI data, specifically assessing correlations in activation between regions, revealed several brain networks involved in the M4 modulation of amphetamine-induced brain activation, including the NAS and retrosplenial cortex with motor cortex, hippocampus, and medial thalamus. Using in vivo microdialysis, we found that VU0152100 reversed amphetamine-induced increases in extracellular dopamine levels in NAS and CP. The present data are consistent with an antipsychotic drug-like profile of activity for VU0152100. Taken together, these data support the development of selective M4 PAMs as a new approach to the treatment of psychosis and cognitive impairments associated with psychiatric disorders such as schizophrenia.
Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats.[Pubmed:18772318]
J Pharmacol Exp Ther. 2008 Dec;327(3):941-53.
Previous clinical and animal studies suggest that selective activators of M(1) and/or M(4) muscarinic acetylcholine receptors (mAChRs) have potential as novel therapeutic agents for treatment of schizophrenia and Alzheimer's disease. However, highly selective centrally penetrant activators of either M(1) or M(4) have not been available, making it impossible to determine the in vivo effects of selective activation of these receptors. We previously identified VU10010 [3-amino-N-(4-chlorobenzyl)-4, 6-dimethylthieno[2,3-b]pyridine-2-carboxamide] as a potent and selective allosteric potentiator of M(4) mAChRs. However, unfavorable physiochemical properties prevented use of this compound for in vivo studies. We now report that chemical optimization of VU10010 has afforded two centrally penetrant analogs, VU0152099 [3-amino-N-(benzo[d][1,3]dioxol-5-ylmethyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide] and VU0152100 [3-amino-N-(4-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide], that are potent and selective positive allosteric modulators of M(4). VU0152099 and VU0152100 had no agonist activity but potentiated responses of M(4) to acetylcholine. Both compounds were devoid of activity at other mAChR subtypes or at a panel of other GPCRs. The improved physiochemical properties of VU0152099 and VU0152100 allowed in vivo dosing and evaluation of behavioral effects in rats. Interestingly, these selective allosteric potentiators of M(4) reverse amphetamine-induced hyperlocomotion in rats, a model that is sensitive to known antipsychotic agents and to nonselective mAChR agonists. This is consistent with the hypothesis that M(4) plays an important role in regulating midbrain dopaminergic activity and raises the possibility that positive allosteric modulation of M(4) may mimic some of the antipsychotic-like effects of less selective mAChR agonists.


