SuprofenSuprofen is a non-steroidal anti-inflammatory drug (NSAID). CAS# 40828-46-4 |
- Vandetanib (ZD6474)
Catalog No.:BCC3883
CAS No.:443913-73-3
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
- Crenolanib (CP-868596)
Catalog No.:BCC3671
CAS No.:670220-88-9
- Apatinib
Catalog No.:BCC5099
CAS No.:811803-05-1
- Flumatinib mesylate
Catalog No.:BCC3970
CAS No.:895519-91-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40828-46-4 | SDF | Download SDF |
| PubChem ID | 5359 | Appearance | Powder |
| Formula | C14H12O3S | M.Wt | 260.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (384.16 mM) *"≥" means soluble, but saturation unknown. | ||
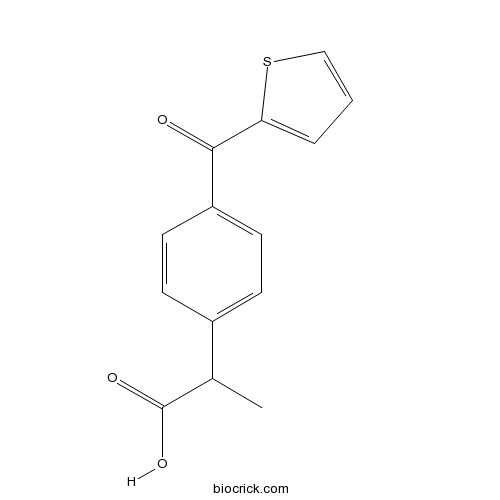
| Chemical Name | 2-[4-(thiophene-2-carbonyl)phenyl]propanoic acid | ||
| SMILES | CC(C1=CC=C(C=C1)C(=O)C2=CC=CS2)C(=O)O | ||
| Standard InChIKey | MDKGKXOCJGEUJW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Suprofen is a non-steroidal anti-inflammatory drug (NSAID).
Target: PGE synthase
Suprofen is an NSAID.Suprofen is an ibuprofen-type anti-inflammatory analgesic and antipyretic. It inhibits prostaglandin synthesis and has been proposed as an anti-arthritic. suprofen was clinically effective but the differential suppression of prostanoids favors 200mg which spares 6-keto PGF1a [1, 2]. References: | |||||

Suprofen Dilution Calculator

Suprofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8416 mL | 19.2079 mL | 38.4157 mL | 76.8315 mL | 96.0393 mL |
| 5 mM | 0.7683 mL | 3.8416 mL | 7.6831 mL | 15.3663 mL | 19.2079 mL |
| 10 mM | 0.3842 mL | 1.9208 mL | 3.8416 mL | 7.6831 mL | 9.6039 mL |
| 50 mM | 0.0768 mL | 0.3842 mL | 0.7683 mL | 1.5366 mL | 1.9208 mL |
| 100 mM | 0.0384 mL | 0.1921 mL | 0.3842 mL | 0.7683 mL | 0.9604 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Suprofen is a non-steroidal anti-inflammatory drug (NSAID).
- MDL 72222
Catalog No.:BCC6733
CAS No.:40796-97-2
- H-D-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2900
CAS No.:4079-64-5
- PD 146176
Catalog No.:BCC7504
CAS No.:4079-26-9
- FPL 55712
Catalog No.:BCC7310
CAS No.:40785-97-5
- 1,7-Diepi-8,15-cedranediol
Catalog No.:BCN5460
CAS No.:40768-81-8
- CaCCinh-A01
Catalog No.:BCC6314
CAS No.:407587-33-1
- (+)-Nerolidol
Catalog No.:BCC8219
CAS No.:142-50-7
- (-)-Bicuculline methiodide
Catalog No.:BCC7387
CAS No.:40709-69-1
- Actinine
Catalog No.:BCN1744
CAS No.:407-64-7
- O-Phospho-L-serine
Catalog No.:BCC6578
CAS No.:407-41-0
- Taxifolin 3-O-beta-D-xylopyranoside
Catalog No.:BCN5458
CAS No.:40672-47-7
- Cornoside
Catalog No.:BCN7575
CAS No.:40661-45-8
- 3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane
Catalog No.:BCN7165
CAS No.:408324-00-5
- 3,5-Dihydroxy-1,7-bis(3,4-dihydroxyphenyl)heptane
Catalog No.:BCN7494
CAS No.:408324-01-6
- H-Tyr-OEt.HCl
Catalog No.:BCC3125
CAS No.:4089-07-0
- Sinoacutine
Catalog No.:BCN5461
CAS No.:4090-18-0
- 3,4-Dimethoxycinnamyl alcohol
Catalog No.:BCN6499
CAS No.:40918-90-9
- JNJ 16259685
Catalog No.:BCC7332
CAS No.:409345-29-5
- VU0152100
Catalog No.:BCC4053
CAS No.:409351-28-6
- Taxiresinol
Catalog No.:BCN4637
CAS No.:40951-69-7
- Glycitein
Catalog No.:BCN5896
CAS No.:40957-83-3
- Medioresinol
Catalog No.:BCN5462
CAS No.:40957-99-1
- Desacetylcinobufotalin
Catalog No.:BCC8166
CAS No.:4099-30-3
- H-D-Ala-OBzl.TosOH
Catalog No.:BCC2850
CAS No.:41036-32-2
Mechanism-based inactivation of cytochrome P450 2C9 by tienilic acid and (+/-)-suprofen: a comparison of kinetics and probe substrate selection.[Pubmed:18838506]
Drug Metab Dispos. 2009 Jan;37(1):59-65.
In vitro experiments were conducted to compare k(inact), K(I) and inactivation efficiency (k(inact)/K(I)) of cytochrome P450 (P450) 2C9 by tienilic acid and (+/-)-Suprofen using (S)-flurbiprofen, diclofenac, and (S)-warfarin as reporter substrates. Although the inactivation of P450 2C9 by tienilic acid when (S)-flurbiprofen and diclofenac were used as substrates was similar (efficiency of approximately 9 ml/min/micromol), the inactivation kinetics were characterized by a sigmoidal profile. (+/-)-Suprofen inactivation of (S)-flurbiprofen and diclofenac hydroxylation was also described by a sigmoidal profile, although inactivation was markedly less efficient (approximately 1 ml/min/micromol). In contrast, inactivation of P450 2C9-mediated (S)-warfarin 7-hydroxylation by tienilic acid and (+/-)-Suprofen was best fit to a hyperbolic equation, where inactivation efficiency was moderately higher (10 ml/min/micromol) and approximately 3-fold higher (3 ml/min/micromol), respectively, relative to that of the other probe substrates, which argues for careful consideration of reporter substrate when mechanism-based inactivation of P450 2C9 is assessed in vitro. Further investigations into the increased inactivation seen with tienilic acid relative to that with (+/-)-Suprofen revealed that tienilic acid is a higher affinity substrate with a spectral binding affinity constant (K(s)) of 2 microM and an in vitro half-life of 5 min compared with a K(s) of 21 microM and a 50 min in vitro half-life for (+/-)-Suprofen. Lastly, a close analog of tienilic acid with the carboxylate functionality replaced by an oxirane ring was devoid of inactivation properties, which suggests that an ionic binding interaction with a positively charged residue in the P450 2C9 active site is critical for recognition and mechanism-based inactivation by these close structural analogs.
Biradical vs singlet oxygen photogeneration in suprofen-cholesterol systems.[Pubmed:27559371]
Beilstein J Org Chem. 2016 Jun 14;12:1196-202.
Cholesterol (Ch) is an important lipidic building block and a target for oxidative degradation, which can be induced via free radicals or singlet oxygen ((1)O2). Suprofen (SP) is a nonsteroidal anti-inflammatory drug that contains the 2-benzoylthiophene (BZT) chromophore and has a pi,pi* lowest triplet excited state. In the present work, dyads (S)- and (R)-SP-alpha-Ch (1 and 2), as well as (S)-SP-beta-Ch (3) have been prepared from beta- or alpha-Ch and SP to investigate the possible competition between photogeneration of biradicals and (1)O2, the key mechanistic steps in Ch photooxidation. Steady-state irradiation of 1 and 2 was performed in dichloromethane, under nitrogen, through Pyrex, using a 400 W medium pressure mercury lamp. The spectral analysis of the separated fractions revealed formation of two photoproducts 4 and 5, respectively. By contrast, under the same conditions, 3 did not give rise to any isolable Ch-derived product. These results point to an intramolecular hydrogen abstraction in 1 and 2 from the C7 position of Ch and subsequent C-C coupling of the generated biradicals. Interestingly, 2 was significantly more photoreactive than 1 indicating a clear stereodifferentiation in the photochemical behavior. Transient absorption spectra obtained for 1-3 were very similar and matched that described for the SP triplet excited state (typical bands with maxima at ca. 350 nm and 600 nm). Direct kinetic analysis of the decay traces at 620 nm led to determination of triplet lifetimes that were ca. 4.1 mus for 1 and 2 and 5.8 mus for 3. From these data, the intramolecular quenching rate constants in 1 and 2 were determined as 0.78 x 10(5) s(-1). The capability of dyads 1-3 to photosensitize the production of singlet oxygen was assessed by time-resolved near infrared emission studies in dichloromethane using perinaphthenone as standard. The quantum yields (PhiDelta) were 0.52 for 1 and 2 and 0.56 for 3. In conclusion, SP-alpha-Ch dyads are unique in the sense that they can be used to photogenerate both biradicals and singlet oxygen, thus being able to initiate Ch oxidation from their triplet excited states following either of the two competing mechanistic pathways.
Photodegradation mechanism of nonsteroidal anti-inflammatory drugs containing thiophene moieties: suprofen and tiaprofenic acid.[Pubmed:19719267]
J Phys Chem B. 2009 Aug 13;113(32):11306-13.
The photodegradation of nonsteroid anti-inflammatory drugs Suprofen, 2-[4-(2-thienoyl)phenyl]propionic acid, and tiaprofenic acid, 2-(5-benzoyl-2-thienyl)propanoic acid, is studied by means of density functional theory. Besides the redox properties of the neutral species, we report on absorption spectra and degradation pathways involving excitation, intersystem crossing to the T(1) state, and spontaneous decarboxylation of the deprotonated species of each drug. The energetics and properties of the Suprofen and tiaprofenic acid systems are found to be very similar to those of the highly photolabile benzyl analogue ketoprofen. Mechanisms leading to the formation of a closed-shell decarboxylated ethyl species, as well as peroxyl radicals capable of initiating lipid peroxidation reactions, are discussed.
Differential suppression of menstrual fluid prostaglandin F2a, prostaglandin E2, 6-keto prostaglandin F1a and thromboxane B2 by suprofen in women with primary dysmenorrhea.[Pubmed:17259081]
Prostaglandins Other Lipid Mediat. 2007 Feb;83(1-2):146-53.
Eleven women with primary dysmenorrhea completed a randomized, double-blind, placebo-controlled, three-way cross-over study comparing 200 and 400mg Suprofen. Menstrual fluid volume did not change. Mean+/-S.E.M. menstrual fluid PGF2a was significantly suppressed from 18.9+/-1.9 microg (placebo) to 10.9+/-1.7 and 9.3+/-2.1 microg with 200 and 400 mg Suprofen, respectively (p=<0.005). PGE2 dropped from 7.8+/-0.9 to 4.6+/-0.8 and 4.6+/-1.1 microg (p=<0.05) and TxB2 from 17.5+/-4.3 to 7.5+/-2.9 and 3.6+/-1.3 microg (p=<0.01), respectively. 6-Keto PGF1a was significantly suppressed (2.7+/-0.4 to 1.9+/-0.5 microg, p=<0.025) with only 400 mg Suprofen. Six subjects rated placebo poor and five fair to very good. In contrast, nine rated Suprofen excellent to fair while two rated poor. Thus, Suprofen was clinically effective but the differential suppression of prostanoids favors 200mg which spares 6-keto PGF1a.


