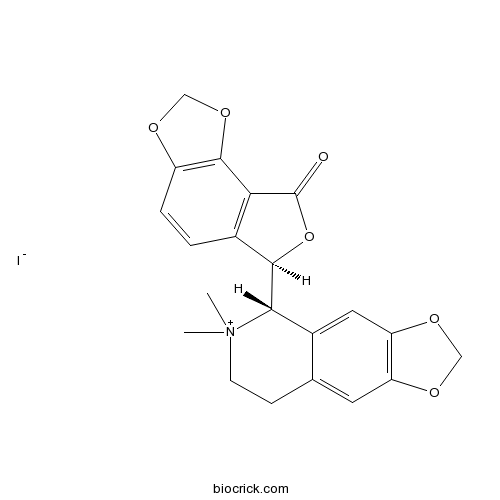
(-)-Bicuculline methiodideWater-soluble GABAA antagonist CAS# 40709-69-1 |
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
- ARN-509
Catalog No.:BCC3724
CAS No.:956104-40-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40709-69-1 | SDF | Download SDF |
| PubChem ID | 104871 | Appearance | Powder |
| Formula | C21H20INO6 | M.Wt | 509.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in water and to 50 mM in DMSO | ||
| Chemical Name | (6R)-6-[(5S)-6,6-dimethyl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]isoquinolin-6-ium-5-yl]-6H-furo[3,4-g][1,3]benzodioxol-8-one;iodide | ||
| SMILES | C[N+]1(CCC2=CC3=C(C=C2C1C4C5=C(C6=C(C=C5)OCO6)C(=O)O4)OCO3)C.[I-] | ||
| Standard InChIKey | HKJKCPKPSSVUHY-GRTNUQQKSA-M | ||
| Standard InChI | InChI=1S/C21H20NO6.HI/c1-22(2)6-5-11-7-15-16(26-9-25-15)8-13(11)18(22)19-12-3-4-14-20(27-10-24-14)17(12)21(23)28-19;/h3-4,7-8,18-19H,5-6,9-10H2,1-2H3;1H/q+1;/p-1/t18-,19+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Methiodide form of classical GABAA receptor antagonist (+)-bicuculline. More water-soluble and stable. Non-GABA receptor-mediated actions reported, including actions on calcium-dependent potassium channels. |

(-)-Bicuculline methiodide Dilution Calculator

(-)-Bicuculline methiodide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9635 mL | 9.8174 mL | 19.6348 mL | 39.2696 mL | 49.087 mL |
| 5 mM | 0.3927 mL | 1.9635 mL | 3.927 mL | 7.8539 mL | 9.8174 mL |
| 10 mM | 0.1963 mL | 0.9817 mL | 1.9635 mL | 3.927 mL | 4.9087 mL |
| 50 mM | 0.0393 mL | 0.1963 mL | 0.3927 mL | 0.7854 mL | 0.9817 mL |
| 100 mM | 0.0196 mL | 0.0982 mL | 0.1963 mL | 0.3927 mL | 0.4909 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Actinine
Catalog No.:BCN1744
CAS No.:407-64-7
- O-Phospho-L-serine
Catalog No.:BCC6578
CAS No.:407-41-0
- Taxifolin 3-O-beta-D-xylopyranoside
Catalog No.:BCN5458
CAS No.:40672-47-7
- Cornoside
Catalog No.:BCN7575
CAS No.:40661-45-8
- IKK-2 inhibitor VIII
Catalog No.:BCC1642
CAS No.:406209-26-5
- ACHP
Catalog No.:BCC6223
CAS No.:406208-42-2
- DSP-4
Catalog No.:BCC7527
CAS No.:40616-75-9
- DMT-T
Catalog No.:BCC2843
CAS No.:40615-39-2
- DMT-Cl
Catalog No.:BCC2799
CAS No.:40615-36-9
- Cirazoline hydrochloride
Catalog No.:BCC6833
CAS No.:40600-13-3
- C34
Catalog No.:BCC5603
CAS No.:40592-88-9
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- (+)-Nerolidol
Catalog No.:BCC8219
CAS No.:142-50-7
- CaCCinh-A01
Catalog No.:BCC6314
CAS No.:407587-33-1
- 1,7-Diepi-8,15-cedranediol
Catalog No.:BCN5460
CAS No.:40768-81-8
- FPL 55712
Catalog No.:BCC7310
CAS No.:40785-97-5
- PD 146176
Catalog No.:BCC7504
CAS No.:4079-26-9
- H-D-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2900
CAS No.:4079-64-5
- MDL 72222
Catalog No.:BCC6733
CAS No.:40796-97-2
- Suprofen
Catalog No.:BCC4943
CAS No.:40828-46-4
- 3,5-Dihydroxy-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)heptane
Catalog No.:BCN7165
CAS No.:408324-00-5
- 3,5-Dihydroxy-1,7-bis(3,4-dihydroxyphenyl)heptane
Catalog No.:BCN7494
CAS No.:408324-01-6
- H-Tyr-OEt.HCl
Catalog No.:BCC3125
CAS No.:4089-07-0
- Sinoacutine
Catalog No.:BCN5461
CAS No.:4090-18-0
Convulsant action of systemically administered glutamate and bicuculline methiodide in immature rats.[Pubmed:11074190]
Epilepsy Res. 2000 Dec;42(2-3):183-9.
Developmental changes of transport of drugs into the brain play an important role in ontogenetic neuropharmacology. Two convulsant drugs with different mechanisms of action (glutamate and bicuculline methiodide) were chosen to demonstrate these changes in developing rats. High dose of glutamate (4 g/kg i.p.) induced both minimal (predominantly clonic) and generalized tonic-clonic seizures in rat pups 7, 12, and 18 days old. In contrast, seizures were only exceptionally observed in 25 and 90 days old animals. Bicuculline methiodide was administered in a dose of 2 or 20 mg/kg i.p. The first sign of bicuculline methiodide action in all age groups was represented by automatisms, a symptomatology never seen after bicuculline hydrochloride administration. Minimal seizures were induced in 12-day-old and in a few 18-day-old and adult rats. Generalized seizures were common after the higher dose of bicuculline methiodide in 7- and 12-day-old rat pups, seldom in 18-day-old ones and never seen in 25-day-old and adult animals. Both glutamate and bicuculline methiodide enter the brain in immature rats but the mechanisms are probably different - glutamate is transported actively through the blood-brain barrier whereas no similar system is known for bicuculline methiodide.
Bicuculline methiodide attenuates hepatic injury and decreases mortality in septic rats: role of cytokines.[Pubmed:15377890]
Shock. 2004 Oct;22(4):347-50.
Bicuculline methiodide attenuates inflammation by inhibiting the production of proinflammatory cytokines, such as tumor necrosis factor-alpha, and by increasing the production of the anti-inflammatory cytokine interleukin-10, both of which play important roles in the pathogenesis of sepsis. The aim of this study was to examine the effects of bicuculline methiodide on sepsis in the cecal ligation and puncture septic-rat model. Cytokine production was measured by enzyme-linked immunosorbent assay. Oxidative stress was assessed by determining serum lipid peroxidation and nitrite levels. Hepatic injury was evaluated by determining the levels of serum aspartate aminotransferase, alkaline phosphatase, and total bilirubin. Mortality was recorded within 24 h. Bicuculline methiodide potently decreased the production of tumor necrosis factor-alpha and interleukin-1beta but increased interleukin-10 in serum. Bicuculline methiodide significantly decreased serum lipid peroxidation and nitrite levels. Further, bicuculline methiodide attenuated hepatic injury and reduced mortality after cecal ligation and puncture. Therefore, the alteration of cytokine production may be involved in the effects of bicuculline methiodide on hepatic injury and mortality in septic rats.
Effect of GABA(A) receptor antagonist bicuculline methiodide on hypotension in endotoxin-intoxicated rats.[Pubmed:15212817]
Toxicology. 2004 Aug 5;200(2-3):213-9.
Gamma-aminobutyric acid (GABA) receptor plays an important regulatory role in human and animal blood pressure; however, whether a GABAergic mechanism is involved in endotoxin intoxication-associated hypotension has never been reported. In vivo effect of the GABA(A) receptor antagonist bicuculline methiodide (BMI) on mean arterial pressure (MAP), heart rate (HR), and serum nitrite levels were investigated in endotoxin (lipopolysaccharide; LPS)-treated rats. BMI increased MAP and decreased HR in LPS-stimulated rats. DOAV ([deamino-Pen, O-Me-Tyr, Arg3]-vasopressin), an arginine vasopressin (AVP) antagonist, reversed the hemodynamic improvement resulted from BMI in LPS-intoxicated rats. Based on a two-factor (BMI treatment by LPS dose) factorial design, BMI reduced the serum nitrite production induced by LPS. DOAV plus BMI, however, did not affect the serum nitrite level in LPS-intoxicated rats. Thus, GABA receptor antagonist BMI might attenuate hypotension in endotoxin intoxication in rats.
Effects of bicuculline methiodide on fast (>200 Hz) electrical oscillations in rat somatosensory cortex.[Pubmed:12163550]
J Neurophysiol. 2002 Aug;88(2):1016-25.
Fast oscillatory activity (more than approximately 200 Hz) has been attracting increasing attention regarding its possible role in both normal brain function and epileptogenesis. Yet, its underlying cellular mechanism remains poorly understood. Our prior investigation of the phenomenon in rat somatosensory cortex indicated that fast oscillations result from repetitive synaptic activation of cortical pyramidal cells originating from GABAergic interneurons (). To test this hypothesis, the effects of topical application of the gamma-aminobutyric acid-A (GABA(A)) antagonist bicuculline methiodide (BMI) on fast oscillations were examined. At subconvulsive concentrations (approximately 10 microM), BMI application resulted in a pronounced enhancement of fast activity, in some trials doubling the number of oscillatory cycles evoked by whisker stimulation. The amplitude and frequency of fast activity were not affected by BMI in a statistically significant fashion. At higher concentrations, BMI application resulted in the emergence of recurring spontaneous slow-wave discharges resembling interictal spikes (IIS) and the eventual onset of seizure. High-pass filtering of the IIS revealed that a burst of fast oscillations accompanied the spontaneous discharge. This activity was present in both the pre- and the postictal regimes, in which its morphology and spatial distribution were largely indistinguishable. These data indicate that fast cortical oscillations do not reflect GABAergic postsynaptic currents. An alternate account consistent with results observed to date is that this activity may instead arise from population spiking in pyramidal cells, possibly mediated by electrotonic coupling in a manner analogous to that underlying 200-Hz ripple in the hippocampus. Additionally, fast oscillations occur within spontaneous epileptiform discharges. However, at least under the present experimental conditions, they do not appear to be a reliable predictor of seizure onset nor an indicator of the seizure focus.
Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia.[Pubmed:16567532]
Diabetes. 2006 Apr;55(4):1080-7.
Hypoglycemia provokes a multifaceted counterregulatory response involving the sympathoadrenal system, stimulation of glucagon secretion, and the hypothalamo-pituitary-adrenal axis that is commonly impaired in diabetes. We examined whether modulation of inhibitory input from gamma-aminobutyric acid (GABA) in the ventromedial hypothalamus (VMH), a major glucose-sensing region within the brain, plays a role in affecting counterregulatory responses to hypoglycemia. Normal Sprague-Dawley rats had carotid artery and jugular vein catheters chronically implanted, as well as bilateral steel microinjection guide cannulas inserted down to the level of the VMH. Seven to 10 days following surgery, the rats were microinjected with artificial extracellular fluid, the GABA(A) receptor agonist muscimol (1 nmol/side), or the GABA(A) receptor antagonist bicuculline methiodide (12.5 pmol/side) before being subjected to a hyperinsulinemic-hypoglycemic (2.5 mmol/l) glucose clamp for 90 min. Following VMH administration of bicuculline methiodide, glucose infusion rates were significantly suppressed, whereas muscimol raised glucose infusion rates significantly compared with controls. Glucagon and epinephrine responses were elevated with the antagonist and suppressed with the agonist compared with controls. Corticosterone responses, however, were unaffected by either administration of the agonist or antagonist into the VMH. These data demonstrate that modulation of the GABAergic system in the VMH alters both glucagon and sympathoadrenal, but not corticosterone, responses to hypoglycemia. Our findings are consistent with the hypothesis that GABAergic inhibitory tone within the VMH can modulate glucose counterregulatory responses.
Differential effects of iontophoretic in vivo application of the GABA(A)-antagonists bicuculline and gabazine in sensory cortex.[Pubmed:16442250]
Hear Res. 2006 Feb;212(1-2):224-35.
We have compared the effects of microiontophoretic application of the GABA(A)-receptor antagonists bicuculline (BIC) and gabazine (SR95531) on responses to pure tones and to sinusoidally amplitude-modulated (AM) tones in cells recorded extracellularly from primary auditory cortex (AI) of Mongolian gerbils. Besides similar effects in increasing spontaneous and stimulus-evoked activity and their duration, both drugs elicited differential effects on spectral tuning and synchronized responses to AM tones. In contrast to gabazine, iontophoresis of the less potent GABA(A)-antagonist BIC often resulted in substantial broadening of frequency tuning for pure tones and an elimination of synchronized responses to AM tones, particularly with high ejecting currents. BIC-induced effects which could not be replicated by application of gabazine were presumably due to the well-documented, non-GABAergic side-effects of BIC on calcium-dependent potassium channels. Our results thus provide strong evidence that GABA(A)-mediated inhibition in AI does not sharpen frequency tuning for pure tones, but rather contributes to the processing of fast temporal modulations of sound envelopes. They also demonstrate that BIC can have effects on neuronal response selectivity which are not due to blockade of GABAergic inhibition. The results have profound implications for microiontophoretic studies of the role of intracortical inhibition in sensory cortex.
Studies on the neuropharmacological activity of bicuculline and related compounds.[Pubmed:1247886]
Brain Res. 1976 Feb 6;102(2):283-99.
Bicuculline and 3 chemical derivatives were assayed on a variety of biological systems. Consistent with reports of studies on other animals, some of these compounds caused convulsions in insects and blocked inhibitory postsynaptic potentials in insect muscle. They all potently inhibited mouse brain acetylcholinesterase. Bicuculline and its analogs inhibited the binding of GABA in vitro to sites in crayfish muscle membranes which have properties of receptor sites; this site of action could explain the activity of bicuculline at arthropod neuromuscular junctions. These compounds, at high concentrations (over 100 muM), also inhibited GABA uptake by mouse brain homogenates at 0 degrees C apparently non-competitively. Bicucine methyl ester inhibited GABA transport by brain at 37 degrees C, consistent with non-specific membrane effects at high concentrations of drug. These and other observations cast doubt upon the specificity of bicuculline-like compounds for action on GABA synapses, especially for in vitro studies at high drug concentrations (over 10 muM). The neuroactivity of low doses of bicuculline is apparently not explained by these in vitro effects, and could very well be due to inhibition of GABA synapses at either receptor or ionophore sites. At physiological conditions of pH and temperature, bicuculline is hydrolyzed at its lactone moiety to the less active compound bicucine; this could lead to underestimates of the biological activity of bicuculline. More stable analogs studied so far are not more potent, however.


