C34TLR4 inhibitor CAS# 40592-88-9 |
- FLAG tag Peptide
Catalog No.:BCC2562
CAS No.:98849-88-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 40592-88-9 | SDF | Download SDF |
| PubChem ID | 91691128 | Appearance | Powder |
| Formula | C17H27NO9 | M.Wt | 389.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TLR4-IN-C34 | ||
| Solubility | Soluble to 50 mM in water and to 100 mM in DMSO | ||
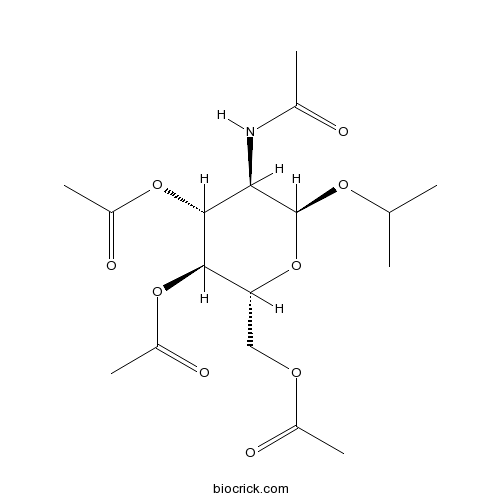
| Chemical Name | [(2R,3S,4R,5R,6S)-5-acetamido-3,4-diacetyloxy-6-propan-2-yloxyoxan-2-yl]methyl acetate | ||
| SMILES | CC(C)OC1C(C(C(C(O1)COC(=O)C)OC(=O)C)OC(=O)C)NC(=O)C | ||
| Standard InChIKey | KMIQMFHPUJUDMC-HHARLNAUSA-N | ||
| Standard InChI | InChI=1S/C17H27NO9/c1-8(2)24-17-14(18-9(3)19)16(26-12(6)22)15(25-11(5)21)13(27-17)7-23-10(4)20/h8,13-17H,7H2,1-6H3,(H,18,19)/t13-,14-,15-,16-,17+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TLR4 inhibitor; attenuates TLR4 signaling in enterocytes and macrophages in vitro, and reduces systemic inflammation in mouse models of endotoxemia and necrotizing enterocolitis (NEC). Also suppresses LPS signaling in human ileum displaying NEC ex-vivo. Displays no significant effects on signaling via TLR2 or TLR9. |

C34 Dilution Calculator

C34 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5681 mL | 12.8403 mL | 25.6805 mL | 51.3611 mL | 64.2013 mL |
| 5 mM | 0.5136 mL | 2.5681 mL | 5.1361 mL | 10.2722 mL | 12.8403 mL |
| 10 mM | 0.2568 mL | 1.284 mL | 2.5681 mL | 5.1361 mL | 6.4201 mL |
| 50 mM | 0.0514 mL | 0.2568 mL | 0.5136 mL | 1.0272 mL | 1.284 mL |
| 100 mM | 0.0257 mL | 0.1284 mL | 0.2568 mL | 0.5136 mL | 0.642 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- SB590885
Catalog No.:BCC4392
CAS No.:405554-55-4
- Cyclapolin 9
Catalog No.:BCC7571
CAS No.:40533-25-3
- Dadahol A
Catalog No.:BCN5457
CAS No.:405281-76-7
- NFPS
Catalog No.:BCC7484
CAS No.:405225-21-0
- Dovitinib (TKI-258, CHIR-258)
Catalog No.:BCC1169
CAS No.:405169-16-6
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- Besifloxacin HCl
Catalog No.:BCC4764
CAS No.:405165-61-9
- Drechslerine D
Catalog No.:BCN7502
CAS No.:405157-88-2
- Drechslerine A
Catalog No.:BCN7561
CAS No.:405157-84-8
- Salubrinal
Catalog No.:BCC4843
CAS No.:405060-95-9
- Cirazoline hydrochloride
Catalog No.:BCC6833
CAS No.:40600-13-3
- DMT-Cl
Catalog No.:BCC2799
CAS No.:40615-36-9
- DMT-T
Catalog No.:BCC2843
CAS No.:40615-39-2
- DSP-4
Catalog No.:BCC7527
CAS No.:40616-75-9
- ACHP
Catalog No.:BCC6223
CAS No.:406208-42-2
- IKK-2 inhibitor VIII
Catalog No.:BCC1642
CAS No.:406209-26-5
- Cornoside
Catalog No.:BCN7575
CAS No.:40661-45-8
- Taxifolin 3-O-beta-D-xylopyranoside
Catalog No.:BCN5458
CAS No.:40672-47-7
- O-Phospho-L-serine
Catalog No.:BCC6578
CAS No.:407-41-0
- Actinine
Catalog No.:BCN1744
CAS No.:407-64-7
- (-)-Bicuculline methiodide
Catalog No.:BCC7387
CAS No.:40709-69-1
- (+)-Nerolidol
Catalog No.:BCC8219
CAS No.:142-50-7
Glycosyl Phosphatidylinositol-Anchored C34 Peptide Derived From Human Immunodeficiency Virus Type 1 Gp41 Is a Potent Entry Inhibitor.[Pubmed:27155865]
J Neuroimmune Pharmacol. 2016 Sep;11(3):601-10.
Lipid rafts of the plasma membrane have been shown to be gateways for HIV-1 budding and entry. In nature, many glycosyl-phosphatidylinositol (GPI) anchored proteins are targeted to the lipid rafts. In the present study we constructed two fusion genes, in which C34 peptide or AVF peptide control was genetically linked with a GPI-attachment signal. Recombinant lentiviruses expressing the fusion genes were used to transduce TZM.bl and CEMss-CCR5 cells. Here, we show that with a GPI attachment signal both C34 and AVF are targeted to the lipid rafts through a GPI anchor. GPI-C34, but not GPI-AVF, in transduced TZM.bl cells efficiently blocks the infection of diverse HIV-1 strains of various subtypes. GPI-C34-transduced CEMss-CCR5 cells are totally resistant to HIV-1 infection. Importantly, maximum percentage of inhibition (MPI) by GPI-C34 is comparable to, if not higher than, a very high concentration of soluble C34. Potent blocking by GPI-C34 is likely due to its high local concentration, which allows GPI-C34 to efficiently bind to the prehairpin intermediate and prevent its transition to six helical bundle, thereby interfering with membrane fusion and virus entry. Our findings should have important implications in GPI-anchor-based therapy against HIV-1.
Molecular weight-dependent degradation of D-lactate-containing polyesters by polyhydroxyalkanoate depolymerases from Variovorax sp. C34 and Alcaligenes faecalis T1.[Pubmed:26109003]
Appl Microbiol Biotechnol. 2015 Nov;99(22):9555-63.
Polyhydroxyalkanoate depolymerase derived from Variovorax sp. C34 (PhaZVs) was identified as the first enzyme that is capable of degrading isotactic P[67 mol% (R)-lactate(LA)-co-(R)-3-hydroxybutyrate(3HB)] [P(D-LA-co-D-3HB)]. This study aimed at analyzing the monomer sequence specificity of PhaZVs for hydrolyzing P(LA-co-3HB) in comparison with a P(3HB) depolymerase from Alcaligenes faecalis T1 (PhaZAf) that did not degrade the same copolymer. Degradation of P(LA-co-3HB) by action of PhaZVs generated dimers, 3HB-3HB, 3HB-LA, LA-3HB, and LA-LA, and the monomers, suggesting that PhaZVs cleaved the linkages between LA and 3HB units and between LA units. To provide a direct evidence for the hydrolysis of these sequences, the synthetic methyl trimers, 3HB-3HB-3HB, LA-LA-3HB, LA-3HB-LA, and 3HB-LA-LA, were treated with the PhaZs. Unexpectedly, not only PhaZVs but also PhaZAf hydrolyzed all of these substrates, namely PhaZAf also cleaved LA-LA linkage. Considering the fact that both PhaZs did not degrade P[(R)-LA] (PDLA) homopolymer, the cleavage capability of LA-LA linkage by PhaZs was supposed to depend on the length of the LA-clustering region in the polymer chain. To test this hypothesis, PDLA oligomers (6 to 40 mer) were subjected to the PhaZ assay, revealing that there was an inverse relationship between molecular weight of the substrates and their hydrolysis efficiency. Moreover, PhaZVs exhibited the degrading activity toward significantly longer PDLA oligomers compared to PhaZAf. Therefore, the cleaving capability of PhaZs used here toward the D-LA-based polymers containing the LA-clustering region was strongly associated with the substrate length, rather than the monomer sequence specificity of the enzyme.
Molecular dynamics studies of the inhibitor C34 binding to the wild-type and mutant HIV-1 gp41: inhibitory and drug resistant mechanism.[Pubmed:25393106]
PLoS One. 2014 Nov 13;9(11):e111923.
Mutations on NHR (N-terminal heptad repeat) associated with resistance to fusion inhibitor were observed. In addition, mutations on CHR (C-terminal heptad repeat) accompanied NHR mutations of gp41 are noted in many cases, like N43D/S138A double mutation. In this work, we explored the drug resistant mechanism of N43D mutation and the role of S138A second mutation in drug resistance. The binding modes of the wild type gp41 and the two mutants, N43D and N43D/S138A, with the HIV-1 fusion inhibitor C34, a 34-residue peptide mimicking CHR of gp41, were carried out by using molecular dynamics simulations. Based on the MD simulations, N43D mutation affects not only the stability of C34 binding, but also the binding energy of the inhibitor C34. Because N43D mutation may also affect the stable conformation of 6-HB, we introduced S138A second mutation into CHR of gp41 and determined the impact of this mutation. Through the comparative analysis of MD results of the N43D mutant and the N43D/S138A mutant, we found that CHR with S138A mutation shown more favorable affinity to NHR. Compelling differences in structures have been observed for these two mutants, particularly in the binding modes and in the hydrophobic interactions of the CHR (C34) located near the hydrophobic groove of the NHR. Because the conformational stability of 6-HB is important to HIV-1 infection, we suggested a hypothetical mechanism for the drug resistance: N43D single mutation not only impact the binding of inhibitor, but also affect the affinity between NHR and CHR of gp41, thus may reduce the rate of membrane fusion; compensatory mutation S138A would induce greater hydrophobic interactions between NHR and CHR, and render the CHR more compatible to NHR than inhibitors.
The C34 Peptide Fusion Inhibitor Binds to the Six-Helix Bundle Core Domain of HIV-1 gp41 by Displacement of the C-Terminal Helical Repeat Region.[Pubmed:26506247]
Biochemistry. 2015 Nov 17;54(45):6796-805.
The conformational transition of the core domain of HIV-1 gp41 from a prehairpin intermediate to a six-helix bundle is responsible for virus-cell fusion. Several inhibitors which target the N-heptad repeat helical coiled-coil trimer that is fully accessible in the prehairpin intermediate have been designed. One such inhibitor is the peptide C34 derived from the C-heptad repeat of gp41 that forms the exterior of the six-helix bundle. Here, using a variety of biophysical techniques, including dye tagging, size-exclusion chromatography combined with multiangle light scattering, double electron-electron resonance EPR spectroscopy, and circular dichroism, we investigate the binding of C34 to two six-helix bundle mimetics comprising N- and C-heptad repeats either without (core(SP)) or with (core(S)) a short spacer connecting the two. In the case of core(SP), C34 directly exchanges with the C-heptad repeat. For core(S), up to two molecules of C34 bind the six-helix bundle via displacement of the C-heptad repeat. These results suggest that fusion inhibitors such as C34 can target a continuum of transitioning conformational states from the prehairpin intermediate to the six-helix bundle prior to the occurrence of irreversible fusion of viral and target cell membranes.
Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors.[Pubmed:23776545]
PLoS One. 2013 Jun 12;8(6):e65779.
Many inflammatory diseases may be linked to pathologically elevated signaling via the receptor for lipopolysaccharide (LPS), toll-like receptor 4 (TLR4). There has thus been great interest in the discovery of TLR4 inhibitors as potential anti-inflammatory agents. Recently, the structure of TLR4 bound to the inhibitor E5564 was solved, raising the possibility that novel TLR4 inhibitors that target the E5564-binding domain could be designed. We utilized a similarity search algorithm in conjunction with a limited screening approach of small molecule libraries to identify compounds that bind to the E5564 site and inhibit TLR4. Our lead compound, C34, is a 2-acetamidopyranoside (MW 389) with the formula C17H27NO9, which inhibited TLR4 in enterocytes and macrophages in vitro, and reduced systemic inflammation in mouse models of endotoxemia and necrotizing enterocolitis. Molecular docking of C34 to the hydrophobic internal pocket of the TLR4 co-receptor MD-2 demonstrated a tight fit, embedding the pyran ring deep inside the pocket. Strikingly, C34 inhibited LPS signaling ex-vivo in human ileum that was resected from infants with necrotizing enterocolitis. These findings identify C34 and the beta-anomeric cyclohexyl analog C35 as novel leads for small molecule TLR4 inhibitors that have potential therapeutic benefit for TLR4-mediated inflammatory diseases.


