XanthyletinCAS# 553-19-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 553-19-5 | SDF | Download SDF |
| PubChem ID | 65188 | Appearance | Powder |
| Formula | C14H12O3 | M.Wt | 228.24 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
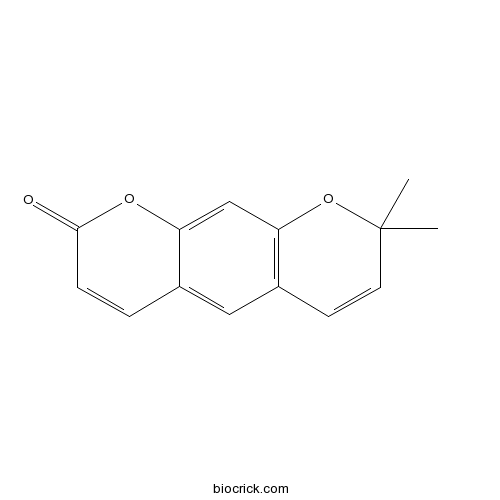
| Chemical Name | 2,2-dimethylpyrano[3,2-g]chromen-8-one | ||
| SMILES | CC1(C=CC2=C(O1)C=C3C(=C2)C=CC(=O)O3)C | ||
| Standard InChIKey | QOTBQNVNUBKJMS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H12O3/c1-14(2)6-5-10-7-9-3-4-13(15)16-11(9)8-12(10)17-14/h3-8H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Xanthyletin exhibits potent inhibition (IC50 values ≤ 4.79 ug/mL) of superoxide anion generation by human nutrophils in response to N-formyl-L-methionyl-L-leucyl-L-phenylalanine/cytochalasin B (fMLP/CB). 2. Xanthyletin inhibits fMLP/CB-induced elastase release with IC50 values ≤ 5.48 ug/mL. 3. Xanthyletin is an inhibitor of symbiotic fungus (Leucoagaricus gongylophorus) of leaf-cutting ant (Atta sexdens rubropilosa). 4. Xanthyletin has anti-inflammatory activity, it displays potent nitric oxide (NO)-reducing activity in microglial cells. |
| Targets | ERK | MMP(e.g.TIMP) | Immunology & Inflammation related |

Xanthyletin Dilution Calculator

Xanthyletin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3814 mL | 21.9068 mL | 43.8135 mL | 87.6271 mL | 109.5338 mL |
| 5 mM | 0.8763 mL | 4.3814 mL | 8.7627 mL | 17.5254 mL | 21.9068 mL |
| 10 mM | 0.4381 mL | 2.1907 mL | 4.3814 mL | 8.7627 mL | 10.9534 mL |
| 50 mM | 0.0876 mL | 0.4381 mL | 0.8763 mL | 1.7525 mL | 2.1907 mL |
| 100 mM | 0.0438 mL | 0.2191 mL | 0.4381 mL | 0.8763 mL | 1.0953 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Thonzonium Bromide
Catalog No.:BCC5636
CAS No.:553-08-2
- Tiamulin
Catalog No.:BCC9179
CAS No.:55297-95-5
- (Z)-Falcarindiol
Catalog No.:BCN8495
CAS No.:55297-87-5
- Atractylodin
Catalog No.:BCN6292
CAS No.:55290-63-6
- Praziquantel
Catalog No.:BCC4829
CAS No.:55268-74-1
- MnTBAP Chloride
Catalog No.:BCC6477
CAS No.:55266-18-7
- Boc-Gln(Xan)-OH
Catalog No.:BCC3385
CAS No.:55260-24-7
- A-674563
Catalog No.:BCC3903
CAS No.:552325-73-2
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- T16Ainh - A01
Catalog No.:BCC6220
CAS No.:552309-42-9
- Vallesiachotamine
Catalog No.:BCN3548
CAS No.:5523-37-5
- Rolapitant
Catalog No.:BCC6441
CAS No.:552292-08-7
- Costunolide
Catalog No.:BCN5740
CAS No.:553-21-9
- Atherosperminine
Catalog No.:BCN8208
CAS No.:5531-98-6
- Soyasaponin II
Catalog No.:BCN1418
CAS No.:55319-36-3
- Petasiphenone
Catalog No.:BCC8100
CAS No.:162616-81-1
- Beclomethasone dipropionate
Catalog No.:BCC4257
CAS No.:5534-09-8
- Vidarabine
Catalog No.:BCC4877
CAS No.:5536-17-4
- Baohuoside II
Catalog No.:BCN2888
CAS No.:55395-07-8
- Hydroxyisoleucine
Catalog No.:BCN8402
CAS No.:55399-93-4
- Lithium carbonate
Catalog No.:BCC7970
CAS No.:554-13-2
- Methazolamide
Catalog No.:BCC2318
CAS No.:554-57-4
- Deferasirox Fe3+ chelate
Catalog No.:BCC1521
CAS No.:554435-83-5
- 8beta-(4-Hydroxytigloyloxy)ovatifolin
Catalog No.:BCN7122
CAS No.:554449-27-3
New benzo[c]phenanthridine and benzenoid derivatives, and other constituents from Zanthoxylum ailanthoides: Effects on neutrophil pro-inflammatory responses.[Pubmed:24232457]
Int J Mol Sci. 2013 Nov 13;14(11):22395-408.
A new benzo[c]phenanthridine, oxynorchelerythrine (1), and two new benzenoid derivatives, methyl 4-(2-hydroxy-4-methoxy-3-methyl-4-oxobutoxy)benzoate (2) and (E)-methyl 4-(4-((Z)-3-methoxy-3-oxoprop-1-enyl)phenoxy)-2-methylbut-2-enoate (3), have been isolated from the twigs of Zanthoxylum ailanthoides, together with 11 known compounds (4-14). The structures of these new compounds were determined through spectroscopic and MS analyses. Among the isolated compounds, decarine (4), (-)-syringaresinol (6), (+)-episesamin (8), glaberide I (9), (-)-dihydrocubebin (10), and Xanthyletin (11) exhibited potent inhibition (IC50 values
Anti-inflammatory principles from the stem and root barks of Citrus medica.[Pubmed:20045968]
Chem Pharm Bull (Tokyo). 2010 Jan;58(1):61-5.
Bioassay-guided investigation of the anti-inflammatory principles from the stem and root barks of Citrus medica L. var. sarcodactylis SWINGLE has led to the isolation of a new coumarin, namely citrumedin-B (1) and thirty known compounds. The anti-inflammatory components were Xanthyletin (2), nordentatin (3), atalantoflavon (4) and lonchocarpol A (5) which displayed potent nitric oxide (NO)-reducing activity in microglial cells. The structure of this new compound was completely elucidated using a combination of 2D NMR techniques (correlation spectroscopy (COSY), nuclear Overhauser effect spectroscopy (NOESY), heteronuclear multiple quantum coherence (HMQC) and heteronuclear multiple bond connectivity (HMBC)) and HR-electrospray ionization (ESI)-MS analyses. The known compounds were identified by comparison of their spectroscopic and physical data with those reported in the literature. These results can be inferred from the treatment of allergic response and inflammatory properties of Citrus medica L. var. sarcodactylis SWINGLE in traditional Chinese medicine.
The butanol fraction of guava (Psidium cattleianum Sabine) leaf extract suppresses MMP-2 and MMP-9 expression and activity through the suppression of the ERK1/2 MAPK signaling pathway.[Pubmed:22211962]
Nutr Cancer. 2012;64(2):255-66.
The leaf extract of guava (Psidium cattleianum Sabine) has traditionally been used for the treatment of diarrhea and diabetes in East Asia and other countries. Recently, the leaf extract has been employed in the therapy of cancer, bacterial infections, and inflammation in experimental models. However, the exact mechanisms of how guava leaf extract inhibits tumor metastasis and invasion are still unknown. In the present study, we investigated in detail the molecular mechanism(s) responsible for the potential antimetastatic and antiinvasive effects of the butanol fraction of guava leaf extract (GBF). Interestingly, we observed for the first time that GBF suppressed both matrix metalloproteinases (MMP)-9 and MMP-2 expression and activity in part through the downregulation of the ERK1/2 activation in lung cancer cells. Also, importantly, the major components of the GBF were identified as d-glucuronic acid, quercetin 3-glucuronide, loganin, and Xanthyletin by LC-ESI-MS/MS. Collectively, our data indicate that the guava leaf could reduce the metastasis of lung cancer cells and therefore suggest that it could be advantageously used to control the metastatic process.
Isolation of xanthyletin, an inhibitor of ants' symbiotic fungus, by high-speed counter-current chromatography.[Pubmed:19296958]
J Chromatogr A. 2009 May 8;1216(19):4307-12.
Xanthyletin, an inhibitor of symbiotic fungus (Leucoagaricus gongylophorus) of leaf-cutting ant (Atta sexdens rubropilosa), as well as suberosin, seselin and xanthoxyletin were isolated from Citrus sinensis grafted on Citrus limonia. A two-phase solvent system composed of hexane/ethanol/acetonitrile/water (10:8:1:1, v/v) was used for the high-speed counter-current chromatographic isolation of Xanthyletin with high yield and over 99% purity as determined by liquid and gas chromatography with mass spectrometry detection. Identifications were performed by UV spectra, IR spectra, (1)H NMR and (13)C NMR.


