Yunnancoronarin ACAS# 162762-93-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 162762-93-8 | SDF | Download SDF |
| PubChem ID | 12187319 | Appearance | Powder |
| Formula | C20H28O2 | M.Wt | 300.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
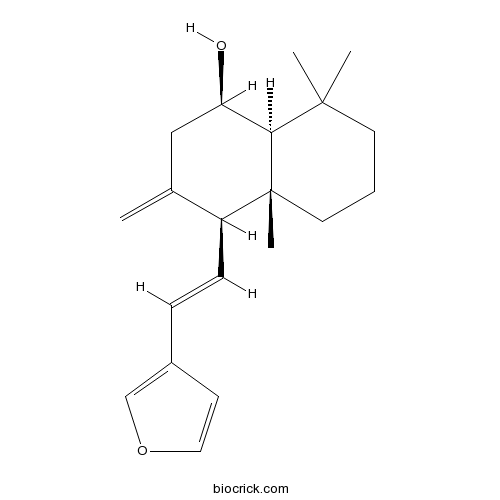
| Chemical Name | (1R,4S,4aR,8aS)-4-[(E)-2-(furan-3-yl)ethenyl]-4a,8,8-trimethyl-3-methylidene-2,4,5,6,7,8a-hexahydro-1H-naphthalen-1-ol | ||
| SMILES | CC1(CCCC2(C1C(CC(=C)C2C=CC3=COC=C3)O)C)C | ||
| Standard InChIKey | UTIGHTZWXIGRIJ-JEQJTPLUSA-N | ||
| Standard InChI | InChI=1S/C20H28O2/c1-14-12-17(21)18-19(2,3)9-5-10-20(18,4)16(14)7-6-15-8-11-22-13-15/h6-8,11,13,16-18,21H,1,5,9-10,12H2,2-4H3/b7-6+/t16-,17+,18-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Yunnancoronarin A shows cytotoxicity against the lung adenocarcinoma cells A549 and leukemia cells K562, with the IC(50) value of 2.20 microM. |

Yunnancoronarin A Dilution Calculator

Yunnancoronarin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3289 mL | 16.6445 mL | 33.2889 mL | 66.5779 mL | 83.2224 mL |
| 5 mM | 0.6658 mL | 3.3289 mL | 6.6578 mL | 13.3156 mL | 16.6445 mL |
| 10 mM | 0.3329 mL | 1.6644 mL | 3.3289 mL | 6.6578 mL | 8.3222 mL |
| 50 mM | 0.0666 mL | 0.3329 mL | 0.6658 mL | 1.3316 mL | 1.6644 mL |
| 100 mM | 0.0333 mL | 0.1664 mL | 0.3329 mL | 0.6658 mL | 0.8322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- WAY-100635
Catalog No.:BCC2053
CAS No.:162760-96-5
- Anemarrhena B
Catalog No.:BCN7592
CAS No.:1627521-95-2
- LDN-214117
Catalog No.:BCC5528
CAS No.:1627503-67-6
- AZD8186
Catalog No.:BCC6470
CAS No.:1627494-13-6
- 1-Hydroxy-2-prenylnaphthalene
Catalog No.:BCN1722
CAS No.:16274-34-3
- 3,4-Dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran
Catalog No.:BCN1539
CAS No.:16274-33-2
- Kaempferol tetraacetate
Catalog No.:BCN1721
CAS No.:16274-11-6
- 6-Deoxyjacareubin
Catalog No.:BCN6573
CAS No.:16265-56-8
- HQL 79
Catalog No.:BCC7703
CAS No.:162641-16-9
- AT 56
Catalog No.:BCC6036
CAS No.:162640-98-4
- AZD3759
Catalog No.:BCC6475
CAS No.:1626387-80-1
- Temsirolimus
Catalog No.:BCC3678
CAS No.:162635-04-3
- PFI-2
Catalog No.:BCC5561
CAS No.:1627676-59-8
- LJI308
Catalog No.:BCC6538
CAS No.:1627709-94-7
- 7-Epi-10-oxo-docetaxel
Catalog No.:BCC5410
CAS No.:162784-72-7
- IEM 1754 dihydrobroMide
Catalog No.:BCC5049
CAS No.:162831-31-4
- 2,3,9,10-Tetrahydroxyberberine
Catalog No.:BCN3550
CAS No.:162854-37-7
- Polygalasaponin V
Catalog No.:BCN2790
CAS No.:162857-65-0
- Polygalaxanthone III
Catalog No.:BCN2354
CAS No.:162857-78-5
- Antibiotic AB 4015B
Catalog No.:BCN1826
CAS No.:162857-79-6
- (S)-3,5-DHPG
Catalog No.:BCC6802
CAS No.:162870-29-3
- Kaempferol-7-O-D-glucopyranoside
Catalog No.:BCN2296
CAS No.:16290-07-6
- 3,4-Dihydroxybenzylamine Hydrobromide
Catalog No.:BCC8280
CAS No.:16290-26-9
- Bis(4-bromophenyl)amine
Catalog No.:BCC8883
CAS No.:16292-17-4
[Studies on chemical constituents from rhizomes of Hedychium chrysoleucum].[Pubmed:19385181]
Zhongguo Zhong Yao Za Zhi. 2009 Jan;34(2):180-2.
OBJECTIVE: To investigate the chemical constituents from the rhizome o fHedychium chrysoleucum. METHOD: The compounds were isolated by column chromatography on silica gel. The structures were identified by NMR, IR and MS analyses. RESULT: Seven compounds were isolated and identified as hedychenone (1), coronarin A (2), Yunnancoronarin A (3), coronarin E (4), beta-sitosterol (5), cryptomeridiol (6), and beta-eudesmol (7). CONCLUSION: Compounds 1-7 were obtained from this plant for the first time.
Cytotoxicity of labdane-type diterpenoids from Hedychium forrestii.[Pubmed:18239312]
Chem Pharm Bull (Tokyo). 2008 Feb;56(2):210-2.
Two new labdane-type diterpenoids, hedyforrestin D (1) and 15-ethoxy-hedyforrestin D (2), and three known compounds, Yunnancoronarin A (4), B (3) and C (5) were isolated from the rhizomes of Hedychium forrestii. The structure of the new diterpenoids was established as 6beta,15xi-dihydroxylabda-8(17),11,13-trien-15,16-olide (1), and 6beta-hydroxy-15xi-ethoxylabda-8(17),11,13-trien-15,16-olide (2) on the basis of spectroscopic analyses. In addition, the isolated compounds were evaluated for their cytotoxicity against the lung adenocarcinoma cells A549 and leukemia cells K562 through 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assays. Of these, compounds 3 and 4 exhibited the most activity with IC(50) values of 0.92 and 2.20 microM, respectively, whereas 5 was inactive against A549 cells and 1 was inactive against both cell lines up to a concentration of 300.81 microM. This shows that both the hydroxy substitution and orientation of unsaturated lactone group in the five-membered ring of C-13 to C-16 seem to play an important role in the anti-tumor activities of human lung adenocarcinoma and leukemia.
Labdane-type diterpenes from Hedychium gardnerianum with potent cytotoxicity against human small cell lung cancer cells.[Pubmed:19960422]
Phytother Res. 2010 Jul;24(7):1009-13.
Seven labdane-type diterpenes, coronarin E, coronarin A, Yunnancoronarin A, yunnancoronarin B, hedyforrestin B, villosin, and hedyforrestin C were isolated from the rhizome of Hedychium gardnerianum and evaluated for cytotoxic activity against human small cell lung cancer (NCI-H187) and non-cancerous Vero cells. The results showed that villosin exhibited potent cytotoxic activity with IC(50) of 0.40 microM, which was higher than that of the drug ellipticine (IC(50) 1.79 microM). Moreover, ellipticine was very toxic to Vero cells (IC(50) 7.47 microM) whereas the toxicity of villosin was undetectable at concentration lower than 166.42 microM. The results have indicated that the lactone ring is essential for high cytotoxic activity and that the presence of a hydroxyl group at the 6 or 7 position causes decrease in activity. The very high cytotoxicity against the NCI-H187 cells and the exceptionally high selectivity index (>416) of villosin suggested that this compound may be used as a potential lead molecule for antitumor therapeutic development.


